NCERT Solutions Class 10 Science Chapter 5 Periodic Classification Of Elements – Here are all the NCERT solutions for Class 10 Science Chapter 5. This solution contains questions, answers, images, explanations of the complete Chapter 5 titled Periodic Classification Of Elements of Science taught in class 10. If you are a student of class 10 who is using NCERT Textbook to study Science, then you must come across Chapter 5 Periodic Classification Of Elements. After you have studied lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification Of Elements in one place.
NCERT Solutions Class 10 Science Chapter 5 Periodic Classification Of Elements
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Science for Class 10 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 5 Periodic Classification Of Elements , Science, Class 10.
| Class | 10 |
| Subject | Science |
| Book | Science |
| Chapter Number | 5 |
| Chapter Name |
Periodic Classification Of Elements |
NCERT Solutions Class 10 Science chapter 5 Periodic Classification Of Elements
Class 10, Science chapter 5, Periodic Classification Of Elements solutions are given below in PDF format. You can view them online or download PDF file for future use.
Periodic Classification Of Elements Download
Did you find NCERT Solutions Class 10 Science chapter 5 Periodic Classification Of Elements helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 10 Science chapter 5 Periodic Classification Of Elements- Video
You can also watch the video solutions of NCERT Class10 Science chapter 5 Periodic Classification Of Elements here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 10 Science chapter 5 Periodic Classification Of Elements In PDF Format
You can also download here the NCERT Solutions Class 10 Science chapter 5 Periodic Classification Of Elements in PDF format.
Click Here to download NCERT Solutions for Class 10 Science chapter 5 Periodic Classification Of Elements
Question & Answer
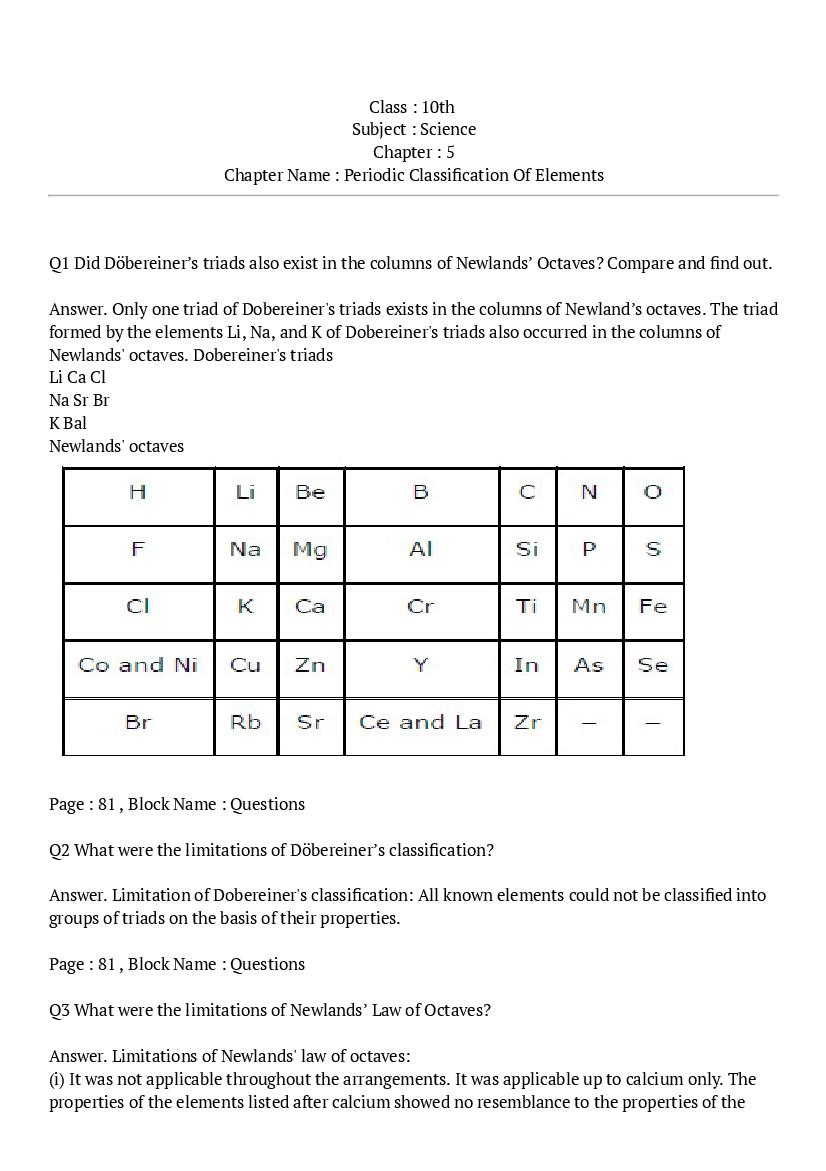
Q.1: Did Döbereiner’s triads also exist in the columns of Newlands’ Octaves? Compare and find out.
Ans : Only one triad of Dobereiner's triads exists in the columns of Newland’s octaves. The triad formed by the elements Li, Na, and K of Dobereiner's triads also occurred in the columns of Newlands' octaves. Dobereiner's triads Li Ca Cl Na Sr Br K Bal Newlands' octaves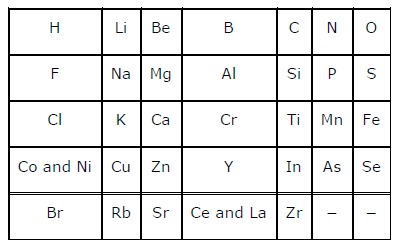
Q.2: What were the limitations of Döbereiner’s classification?
Ans : Limitation of Dobereiner's classification: All known elements could not be classified into groups of triads on the basis of their properties.
Q.3: What were the limitations of Newlands’ Law of Octaves?
Ans : Limitations of Newlands' law of octaves: (i) It was not applicable throughout the arrangements. It was applicable up to calcium only. The properties of the elements listed after calcium showed no resemblance to the properties of the elements above them. (ii) Those elements that were discovered after Newlands' octaves did not follow the law of octaves. (iii) The position of cobalt and nickel in the group of the elements (F, Cl) of different properties could not be explained. (iv) Placing of iron far away from cobalt and nickel, which have similar properties as iron, could also not be explained.
Q.4: Use Mendeléev’s Periodic Table to predict the formulae for the oxides of the following elements: K, C, AI, Si, Ba.
Ans : K is in group 1. Therefore, the oxide will be \( \mathrm{K_{2}O} \). C is in group 4. Therefore, the oxide will be \( \mathrm{CO_{2}} \). A1 is in group 3. Therefore, the oxide will be \( \mathrm{AL_{2}O_{3}} \). Si is in group 4. Therefore, the oxide will be \( \mathrm{SiO_{2}} \). Ba is in group 2. Therefore, the oxide will be \( \mathrm{BaO} \).
Q.5: Besides gallium, which other elements have since been discovered that were left by Mendeléev in his Periodic Table? (any two)
Ans : Scandium and germanium
NCERT / CBSE Book for Class 10 Science
You can download the NCERT Book for Class 10 Science in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 10 Science
- Click here to buy NCERT Book for Class 10 Science
All NCERT Solutions Class 10
- NCERT Solutions for Class 10 English
- NCERT Solutions for Class 10 Hindi
- NCERT Solutions for Class 10 Maths
- NCERT Solutions for Class 10 Science
- NCERT Solutions for Class 10 Social Science
- NCERT Solutions for Class 10 Sanskrit
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 10 Science Chapter 5 Periodic Classification Of Elements. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 10 Science Chapter 5 Periodic Classification Of Elements appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/3ntV72y
https://ift.tt/3eFba9N https://ift.tt/3eFba9N






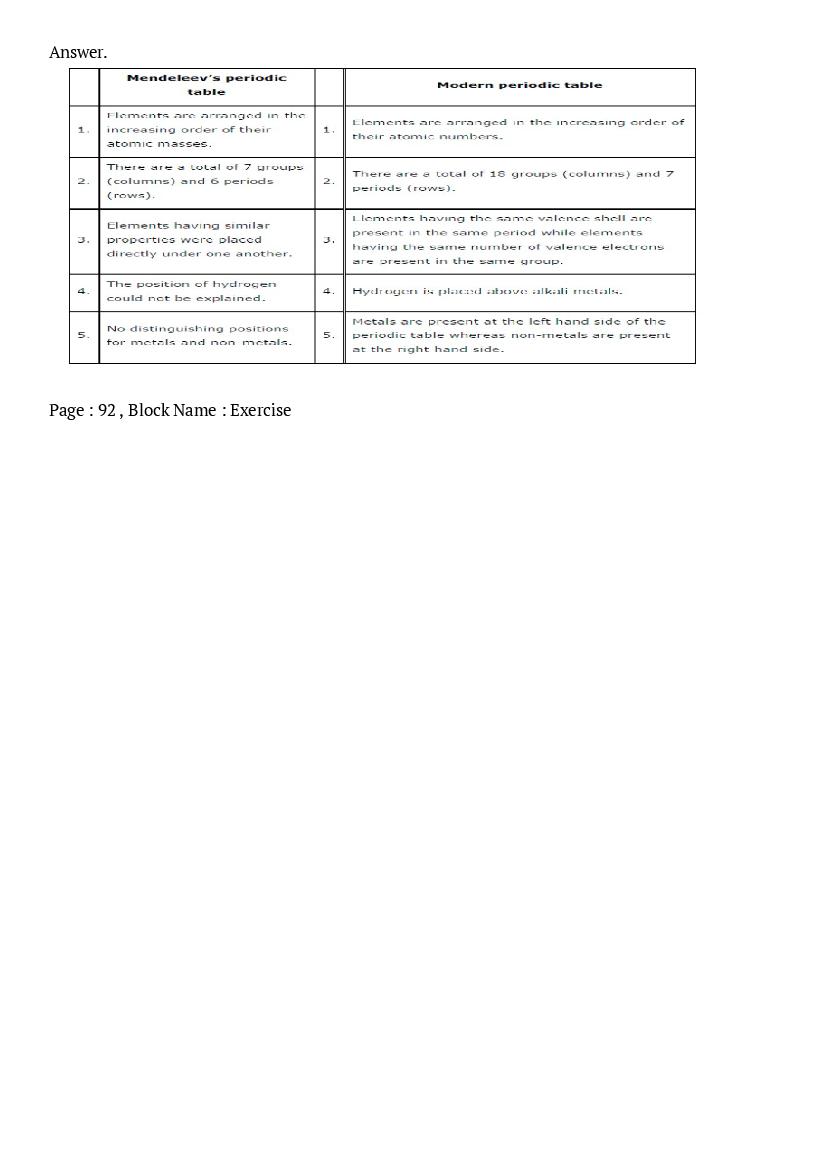
Post a Comment