NCERT Solutions Class 11 Chemistry Chapter 4 Chemical Bond And Molecular Structure.– Here are all the NCERT solutions for Class 11 Chemistry Chapter 4. This solution contains questions, answers, images, explanations of the complete chapter 1 titled Chemical Bond And Molecular Structure taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Chemistry, then you must come across chapter 4 Chemical Bond And Molecular Structure After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bond And Molecular Structure in one place.
NCERT Solutions Class 11 Chemistry Chapter 4 Chemical Bond And Molecular Structure
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Chemistry for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 4 Chemical Bond And Molecular Structure , Chemistry, Class 11.
| Class | 11 |
| Subject | Chemistry |
| Book | Chemistry Part I |
| Chapter Number | 4 |
| Chapter Name |
Chemical Bond And Molecular Structure |
NCERT Solutions Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure
Class 11, Chemistry chapter 4, Chemical Bond And Molecular Structure solutions are given below in PDF format. You can view them online or download PDF file for future use.
Chemical Bond And Molecular Structure
Did you find NCERT Solutions Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure- Video
You can also watch the video solutions of NCERT Class11 Chemistry chapter 4 Chemical Bond And Molecular Structure here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure In PDF Format
You can also download here the NCERT Solutions Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure in PDF format.
Click Here to download NCERT Solutions for Class 11 Chemistry chapter 4 Chemical Bond And Molecular Structure
Question & Answer
Q.1: Explain the formation of a chemical bond.
Ans : A chemical bond is defined as an attractive force that holds the constituents (atoms, ions etc.) together in a chemical species. Various theories have been suggested for the formation of chemical bonds such as the electronic theory, valence shell electron pair repulsion theory, valence bond theory, and molecular orbital theory. A chemical bond formation is attributed to the tendency of a system to attain stability. It was observed that the inertness of noble gases was because of their fully filled outermost orbitals. Hence, it was postulated that the elements having incomplete outermost shells are unstable (reactive). Atoms, therefore, combine with each other and complete their respective octets or duplets to attain the stable configuration of the nearest noble gases. This combination can occur either by sharing of electrons or by transferring one or more electrons from one atom to another. The chemical bond formed as a result of sharing of electrons between atoms is called a covalent bond. An ionic bond is formed as a result of the transference of electrons from one atom to another.
Q.2: Write Lewis symbols for the following atoms and ions: \(\mathrm{S} \text { and } \mathrm{S}^{2-} ; \text { Al and } \mathrm{Al}^{3+} ; \mathrm{H} \text { and } \mathrm{H}^{-}\).
Ans : (i) \(\mathrm{S} \text { and } \mathrm{S}^{2-}\) The number of valence electrons in sulphur is 6.
Q.3: Define octet rule. Write its significance and limitations.
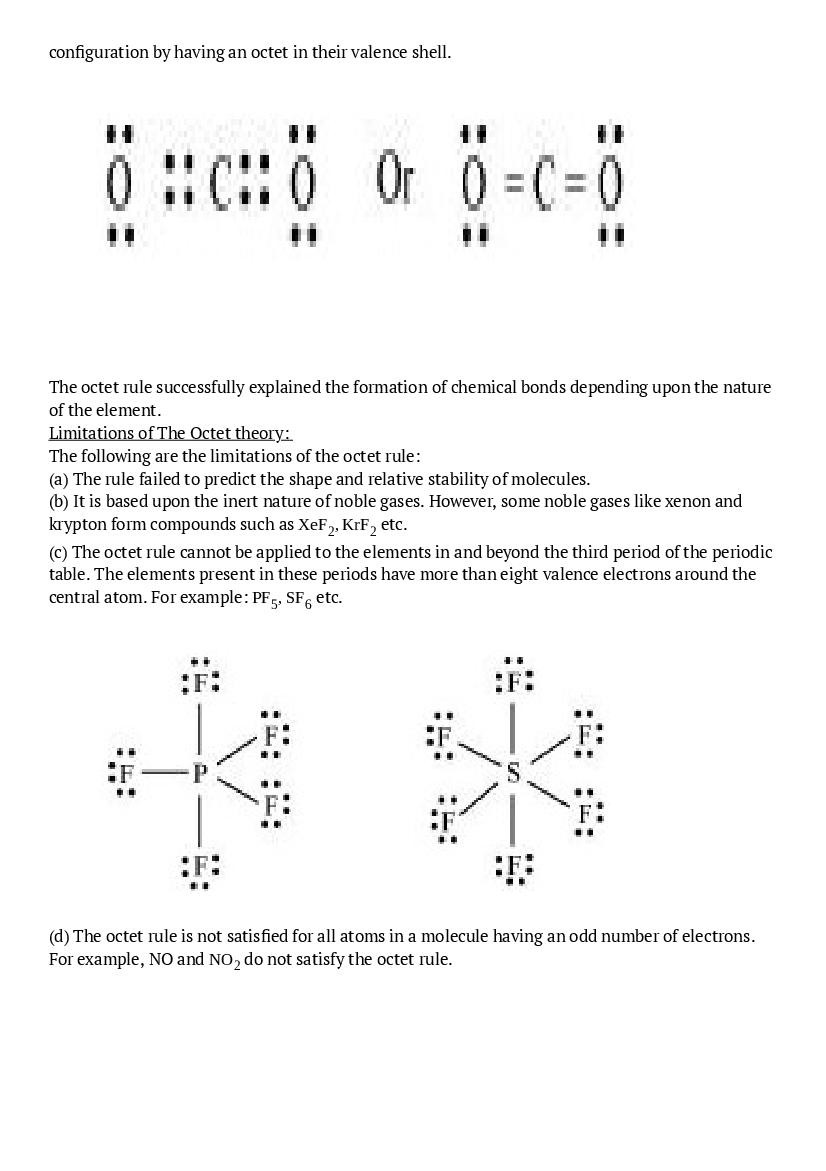
Ans : The octet rule or the electronic theory of chemical bonding was developed by Kossel and Lewis. According to this rule, atoms can combine either by transfer of valence electrons from one atom to another or by sharing their valence electrons in order to attain the nearest noble gas configuration by having an octet in their valence shell.The octet rule successfully explained the formation of chemical bonds depending upon the nature of the element. Limitations of The Octet theory: The following are the limitations of the octet rule: (a) The rule failed to predict the shape and relative stability of molecules. (b) It is based upon the inert nature of noble gases. However, some noble gases like xenon and krypton form compounds such as \(\mathrm{XeF}_{2}, \mathrm{KrF}_{2}\) etc. (c) The octet rule cannot be applied to the elements in and beyond the third period of the periodic table. The elements present in these periods have more than eight valence electrons around the central atom. For example: \(\mathrm{PF}_{5}, \mathrm{SF}_{6}\) etc.
(d) The octet rule is not satisfied for all atoms in a molecule having an odd number of electrons. For example, NO and \(\mathrm{NO}_{2}\) do not satisfy the octet rule.
(e) This rule cannot be applied to those compounds in which the number of electrons surrounding the central atom is less than eight. For example, LiCl, \(\mathrm{BeH}_{2}, \mathrm{AlCl}_{3}\)etc. do not obey the octet rule.
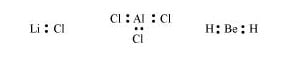
Q.4: Write the favourable factors for the formation of ionic bond.
Ans : An ionic bond is formed by the transfer of one or more electrons from one atom to another. Hence, the formation of ionic bonds depends upon the ease with which neutral atoms can lose or gain electrons. Bond formation also depends upon the lattice energy of the compound formed. Hence, favourable factors for ionic bond formation are as follows: (i) Low ionization enthalpy of metal atom. (ii) High electron gain enthalpy \(\left(\Delta_{\mathrm{eg}} \mathrm{H}\right)\) of a nonmetal atom. (iii) High lattice energy of the compound formed.
Q.5: Discuss the shape of the following molecules using the VSEPR model:
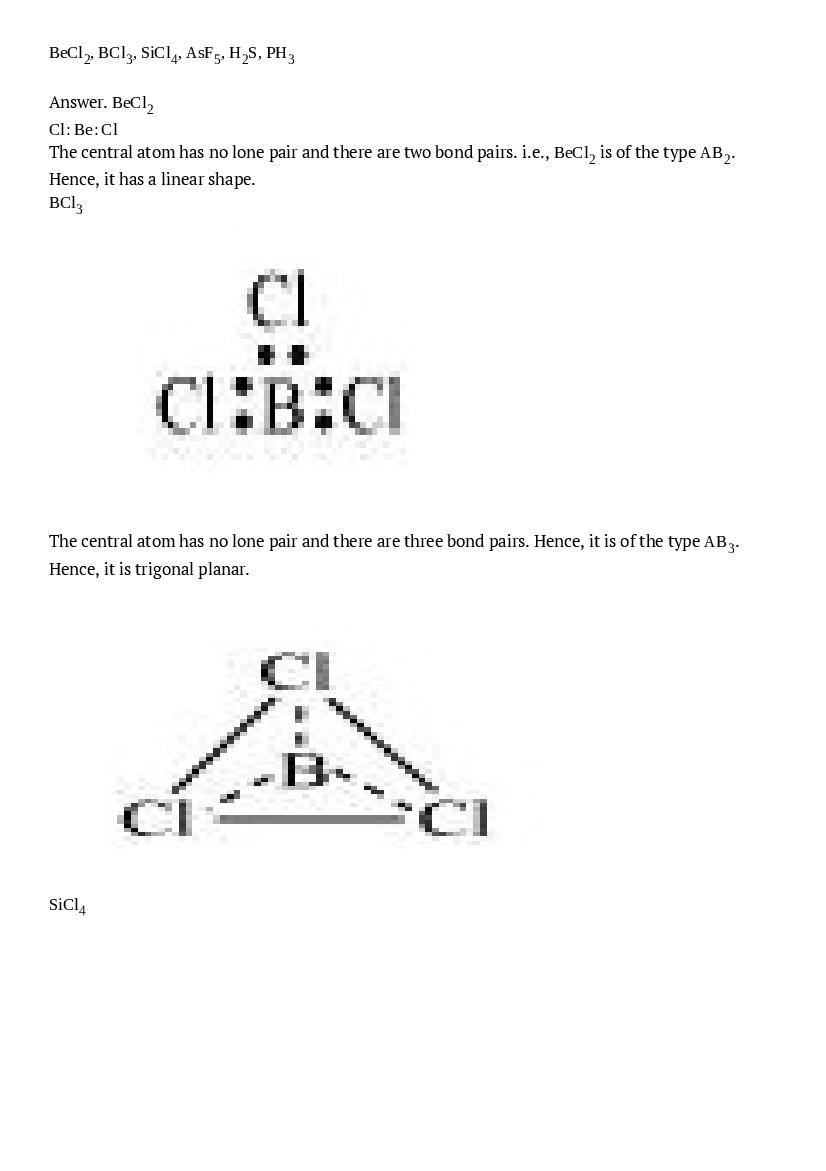
\(\mathrm{BeCl}_{2}, \mathrm{BCl}_{3}, \mathrm{SiCl}_{4}, \mathrm{AsF}_{5}, \mathrm{H}_{2} \mathrm{S}, \mathrm{PH}_{3}\)
Ans : \(\mathrm{BeCl}_{2}\) \(\mathrm{Cl} : \mathrm{Be} : \mathrm{Cl}\) The central atom has no lone pair and there are two bond pairs. i.e., \(\mathrm{BeCl}_{2}\) is of the type \(\mathrm{AB}_{2}\). Hence, it has a linear shape. \(\mathrm{BCl}_{3}\)The central atom has no lone pair and there are three bond pairs. Hence, it is of the type \(\mathrm{AB}_{3}\). Hence, it is trigonal planar.
\(\mathrm{SiCl}_{4}\)
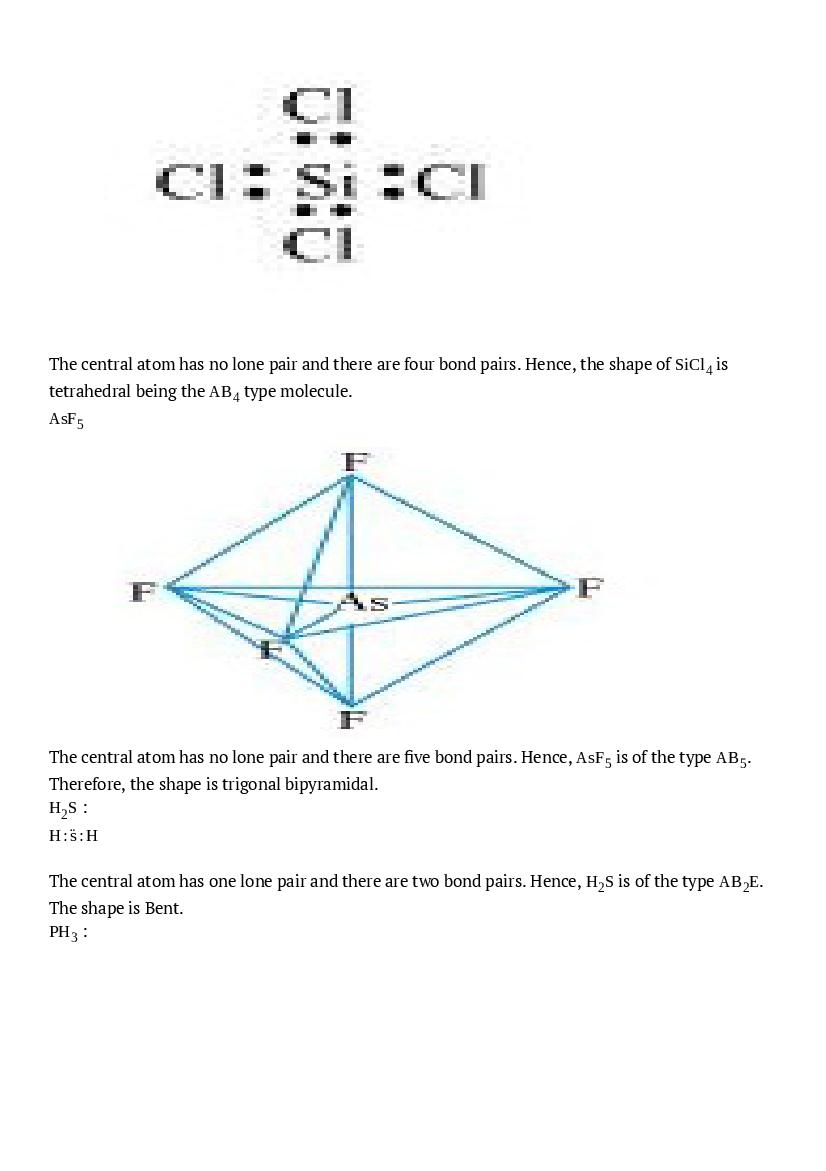
The central atom has no lone pair and there are four bond pairs. Hence, the shape of \(\mathrm{SiCl}_{4}\) is tetrahedral being the \(\mathrm{AB}_{4}\) type molecule. \(\mathrm{AsF}_{5}\)
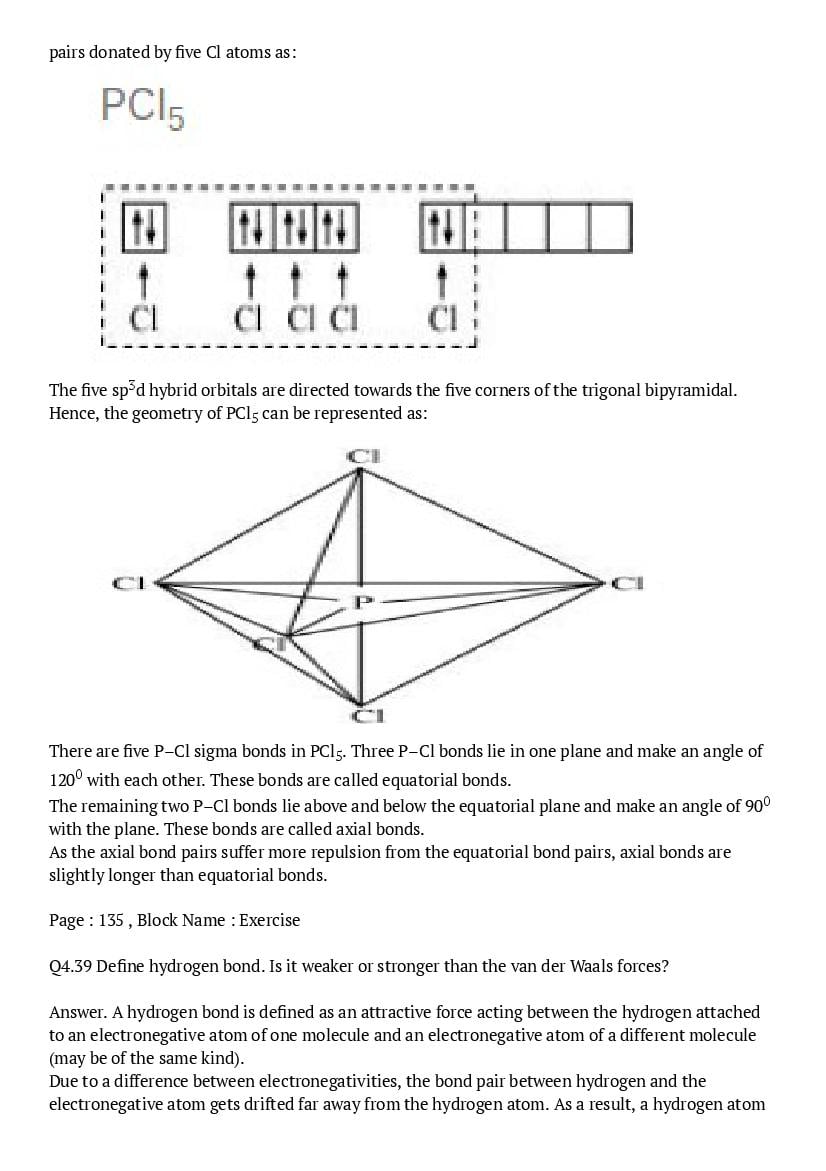
The central atom has no lone pair and there are five bond pairs. Hence, \(\mathrm{AsF}_{5}\) is of the type \(\mathrm{AB}_{5}\). Therefore, the shape is trigonal bipyramidal. \(\mathrm{H}_{2} \mathrm{S}\) : \(\mathrm{H} : \ddot{\mathrm{s}} : \mathrm{H}\) The central atom has one lone pair and there are two bond pairs. Hence, \(\mathrm{H}_{2} \mathrm{S}\) is of the type \(\mathrm{AB}_{2} \mathrm{E}\). The shape is Bent. \(\mathrm{PH}_{3}\) :
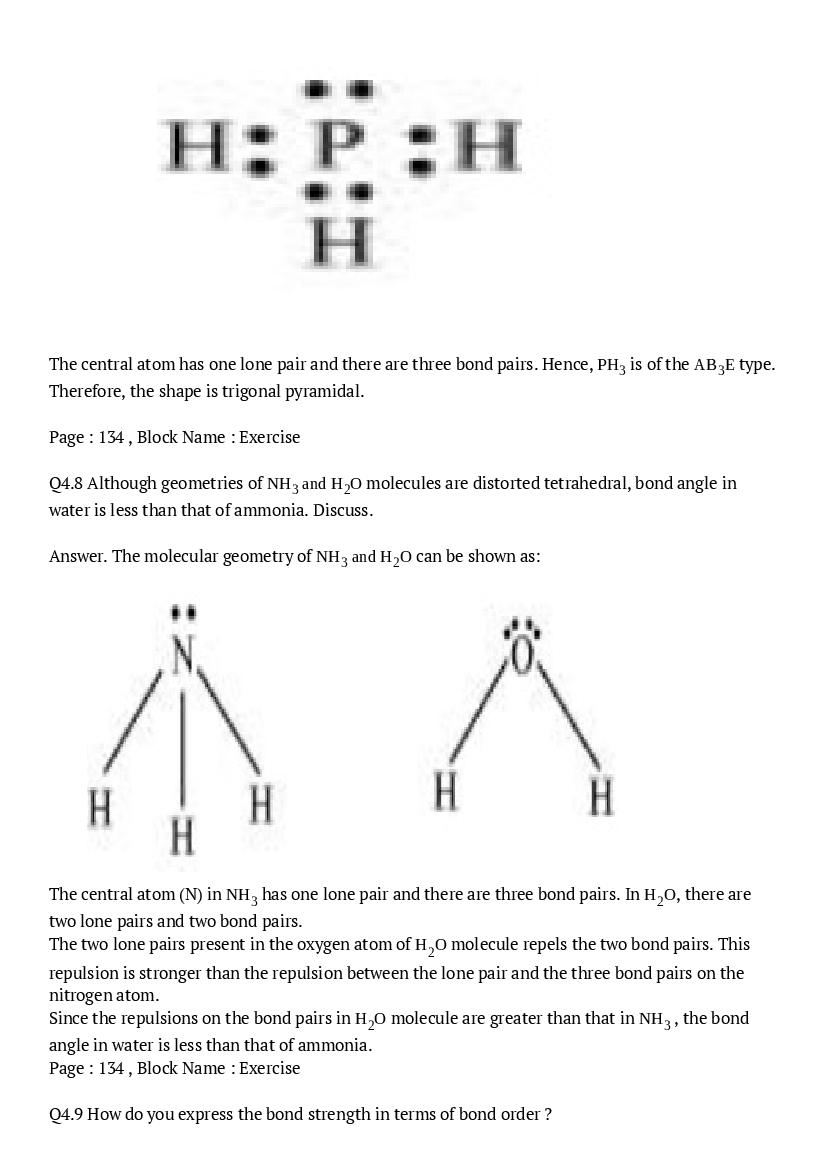
The central atom has one lone pair and there are three bond pairs. Hence, \(\mathrm{PH}_{3}\) is of the \(\mathrm{AB}_{3} \mathrm{E}\) type. Therefore, the shape is trigonal pyramidal.
NCERT / CBSE Book for Class 11 Chemistry
You can download the NCERT Book for Class 11 Chemistry in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Chemistry
- Click here to buy NCERT Book for Class 11 Chemistry
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
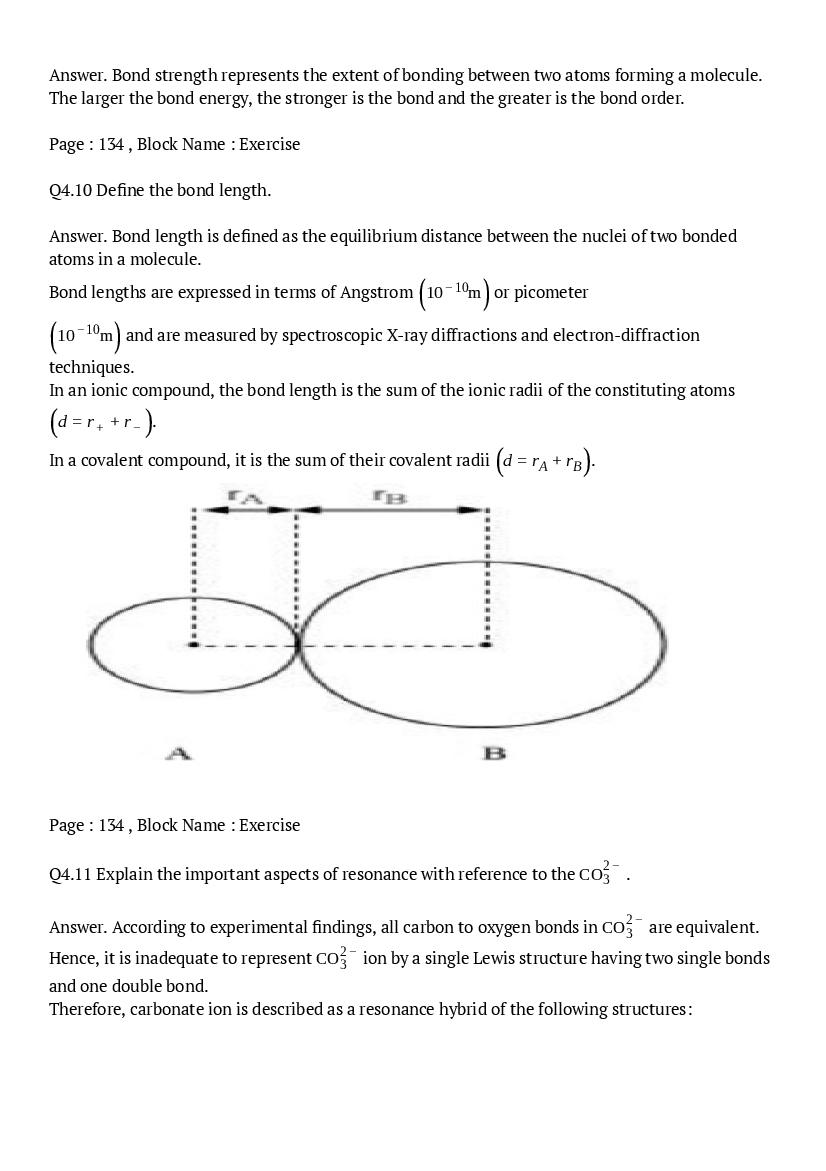
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
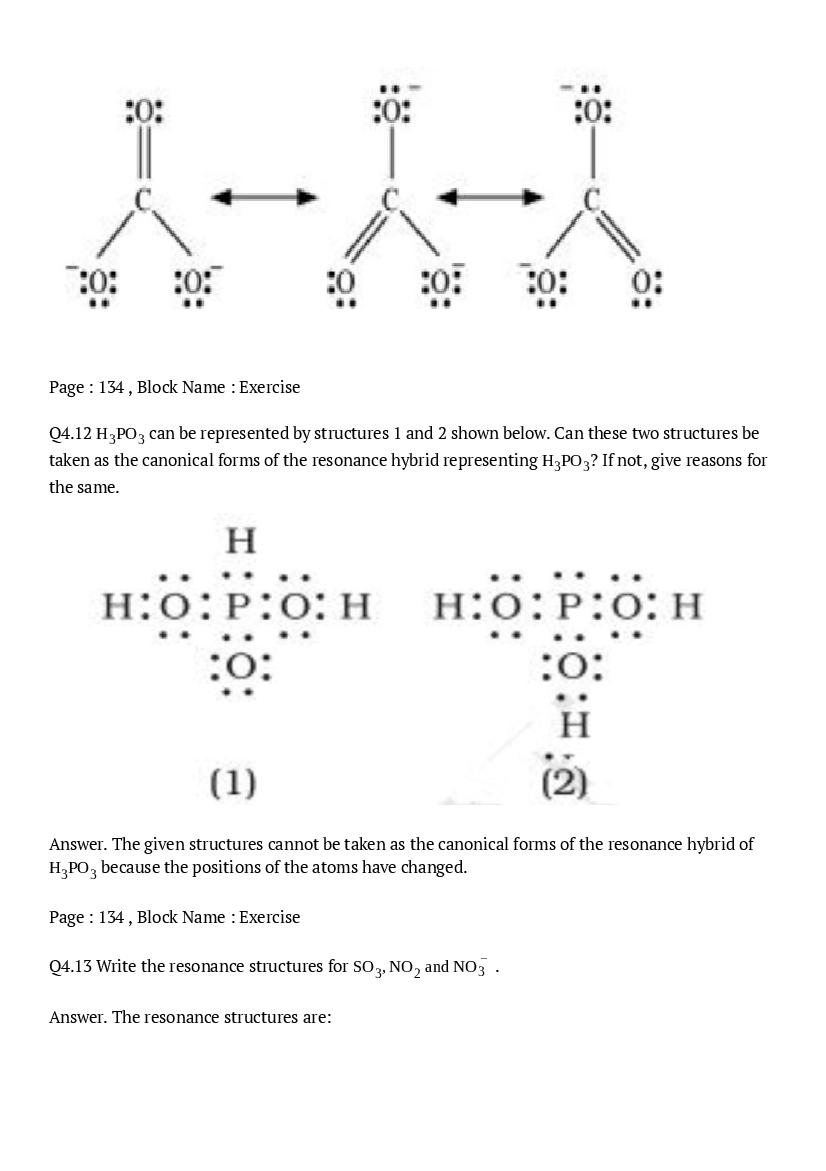
- NCERT Solutions for Class 11 Maths
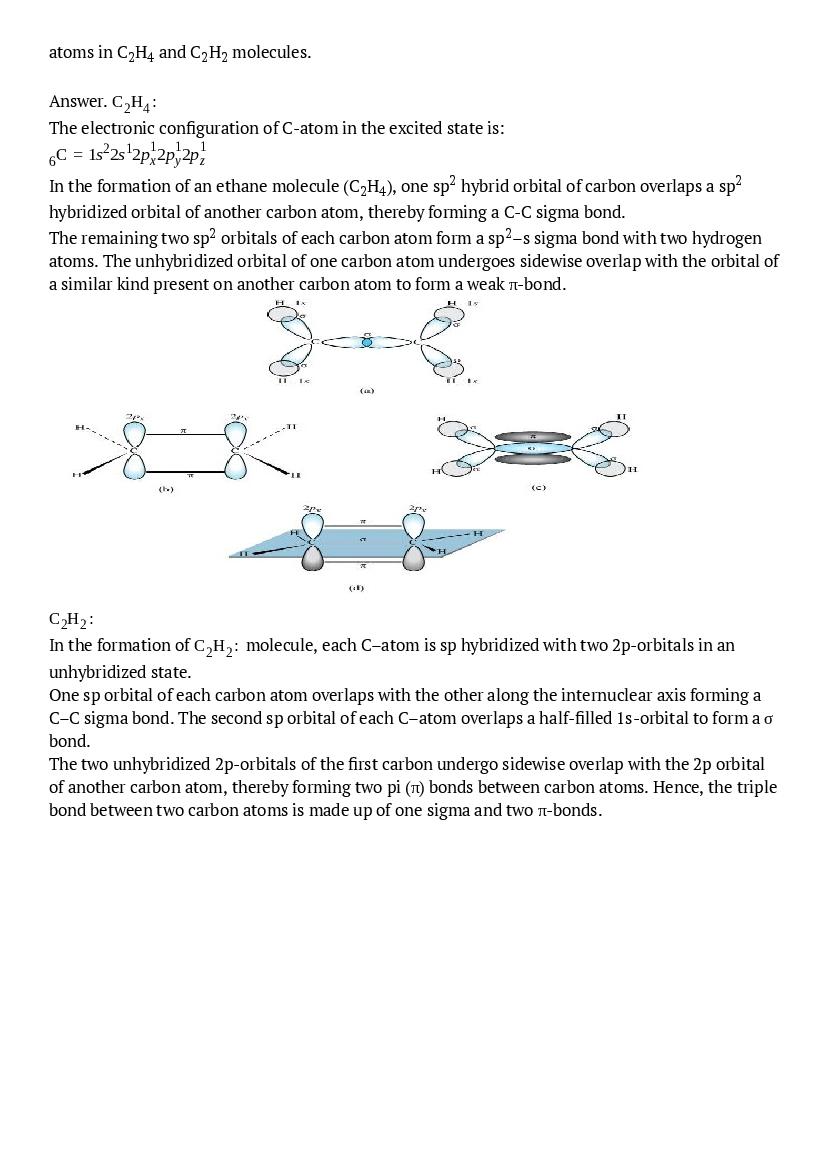
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
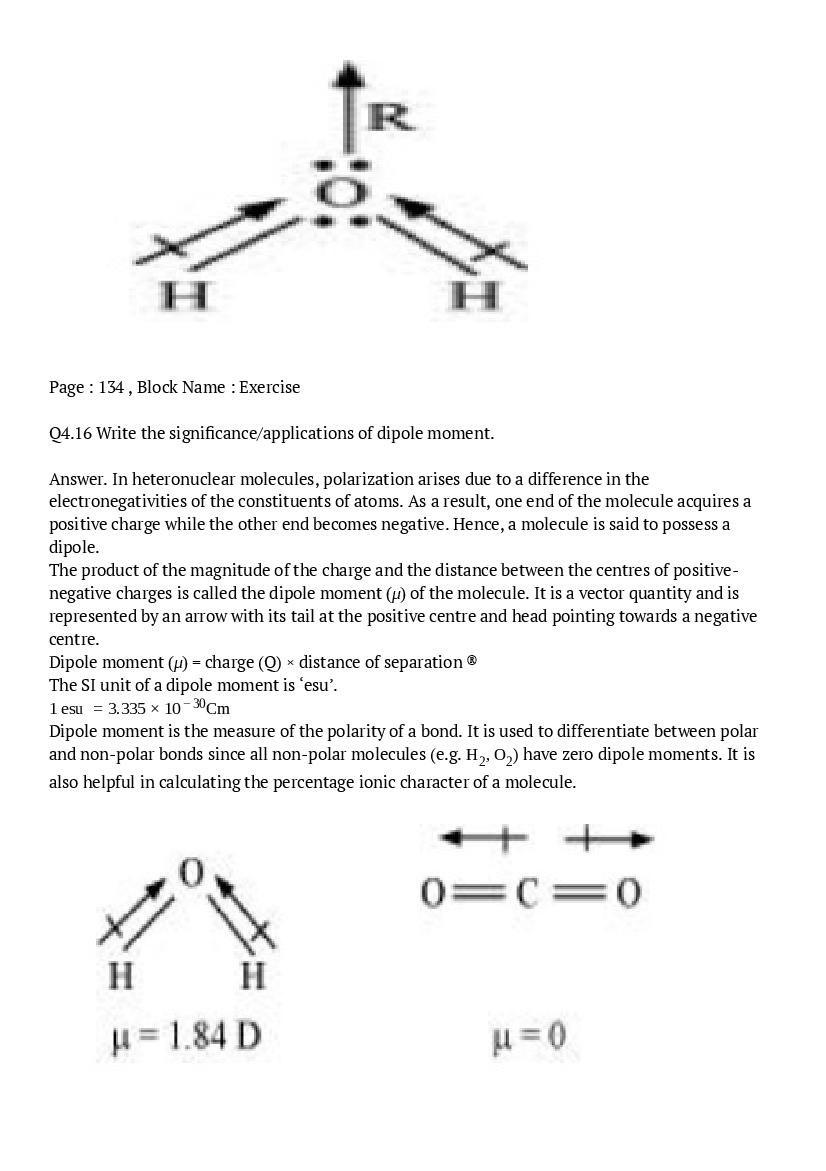
- NCERT Solutions for Class 11 Sociology
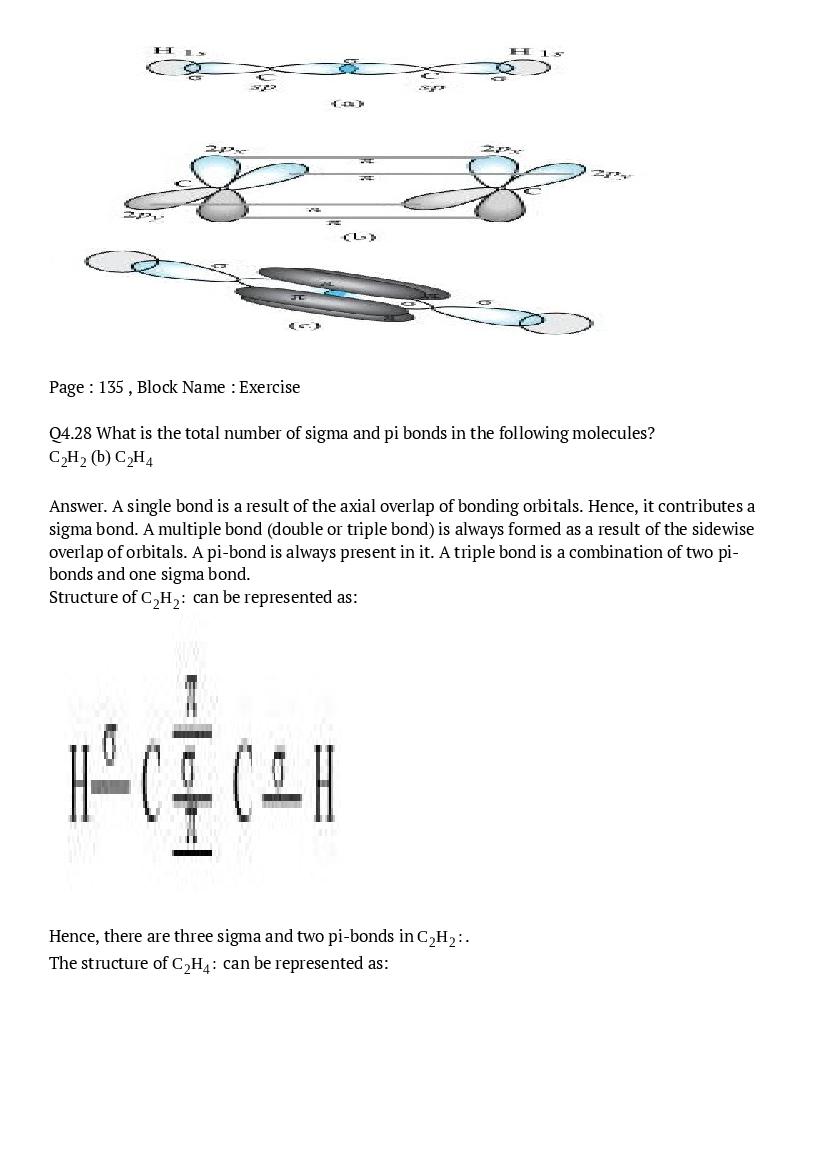
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
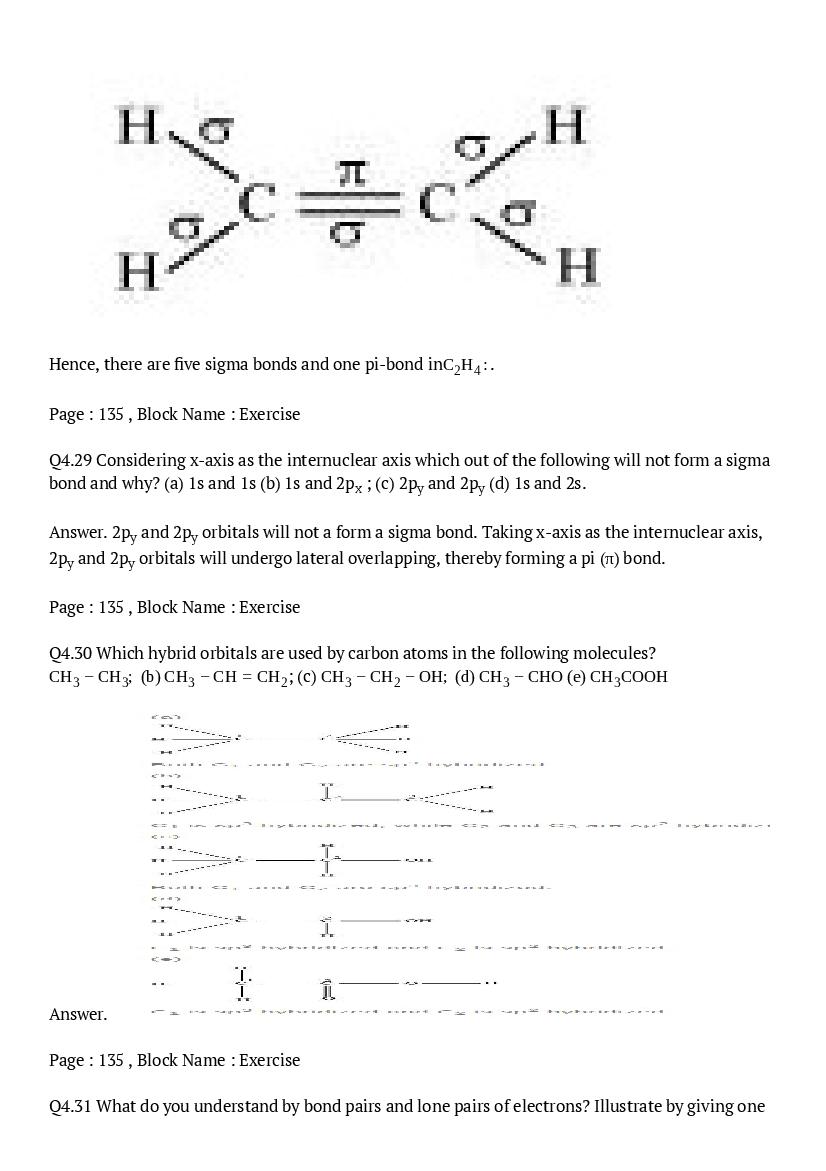
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Chemistry Chapter 4 Chemical Bond And Molecular Structure. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bond And Molecular Structure appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/3gFilRX
https://ift.tt/3aHaYpo https://ift.tt/3aHaYpo










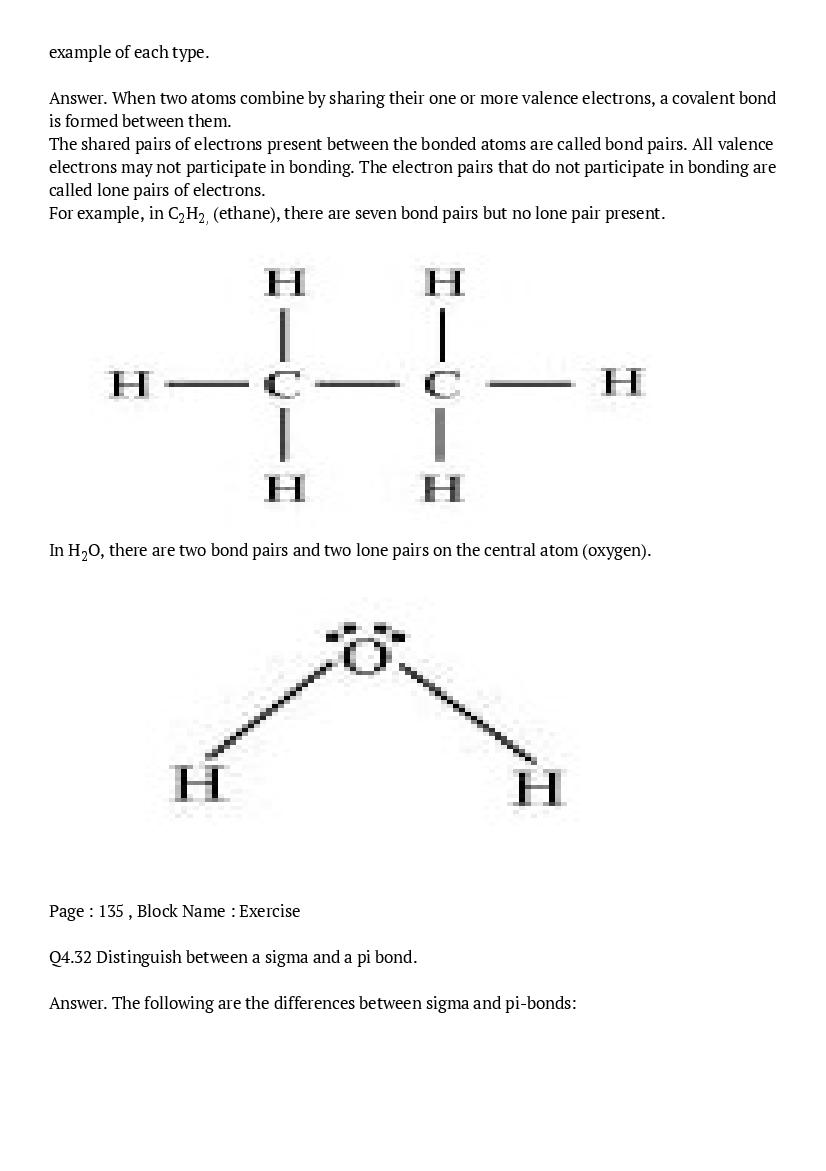




إرسال تعليق