NCERT Solutions Class 11 Chemistry Chapter 11 Hydrogen– Here are all the NCERT solutions for Class 11 Chemistry Chapter 11. This solution contains questions, answers, images, explanations of the complete chapter 1 titled Hydrogen taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Chemistry, then you must come across chapter 11 Hydrogen After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Chemistry Chapter 11 Hydrogen in one place.
NCERT Solutions Class 11 Chemistry Chapter 11 The p-Block element
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Chemistry for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 11 The p-Block element , Chemistry, Class 11.
| Class | 11 |
| Subject | Chemistry |
| Book | Chemistry Part I |
| Chapter Number | 11 |
| Chapter Name |
The p-Block element |
NCERT Solutions Class 11 Chemistry chapter 11 The p-Block element
Class 11, Chemistry chapter 11, The p-Block element solutions are given below in PDF format. You can view them online or download PDF file for future use.
The p-Block element
Did you find NCERT Solutions Class 11 Chemistry chapter 11 The p-Block element helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Chemistry chapter 11 The p-Block element- Video
You can also watch the video solutions of NCERT Class11 Chemistry chapter 11 The p-Block element here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Chemistry chapter 11 The p-Block element In PDF Format
You can also download here the NCERT Solutions Class 11 Chemistry chapter 11 The p-Block element in PDF format.
Click Here to download NCERT Solutions for Class 11 Chemistry chapter 11 The p-Block element
Question & Answer
Q.1: Discuss the pattern of variation in the oxidation states of (i) B to Tl and (ii) C to Pb.
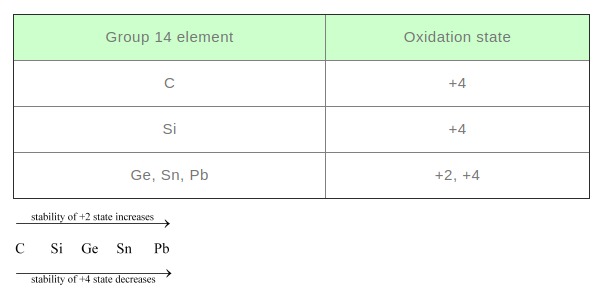
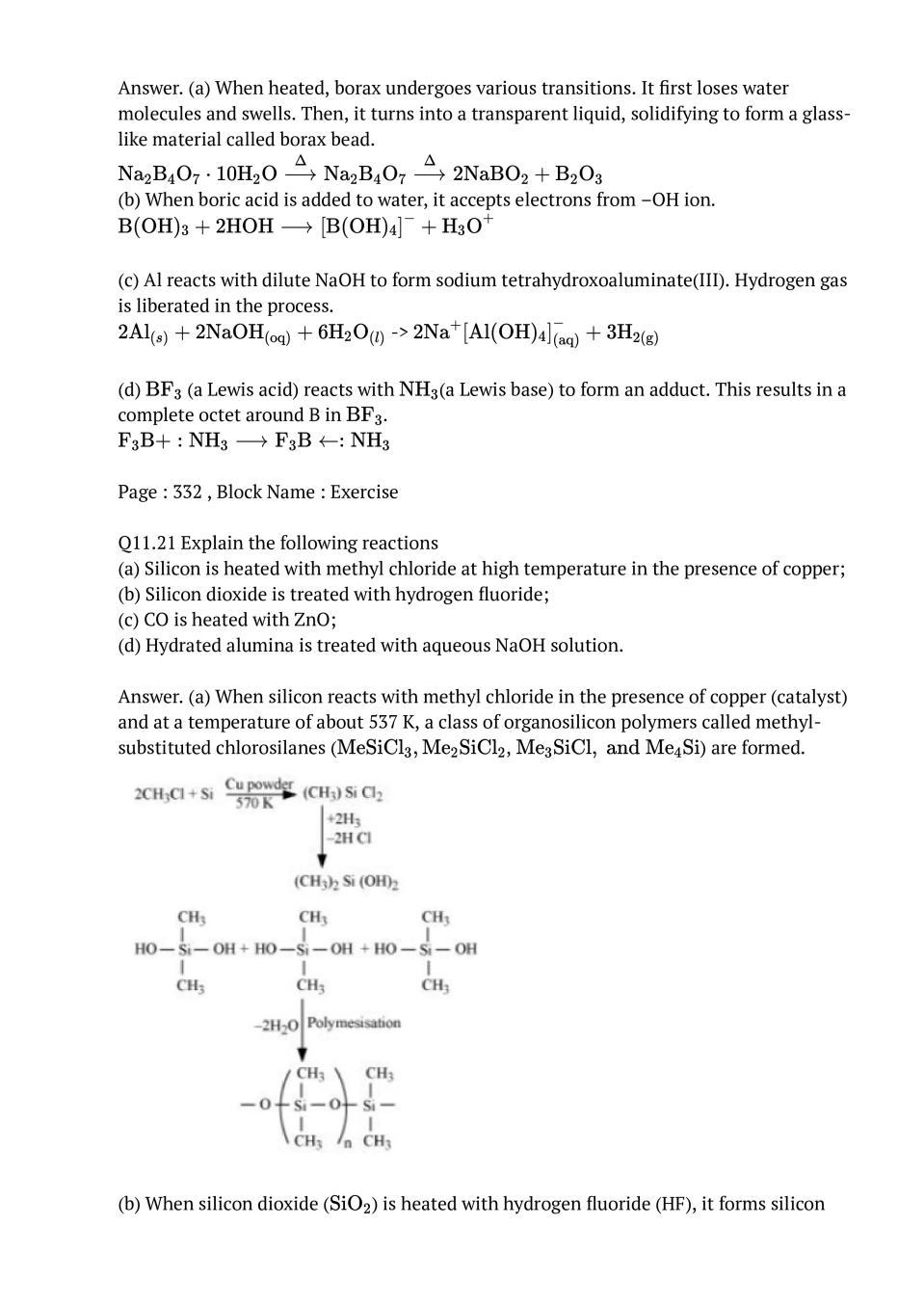
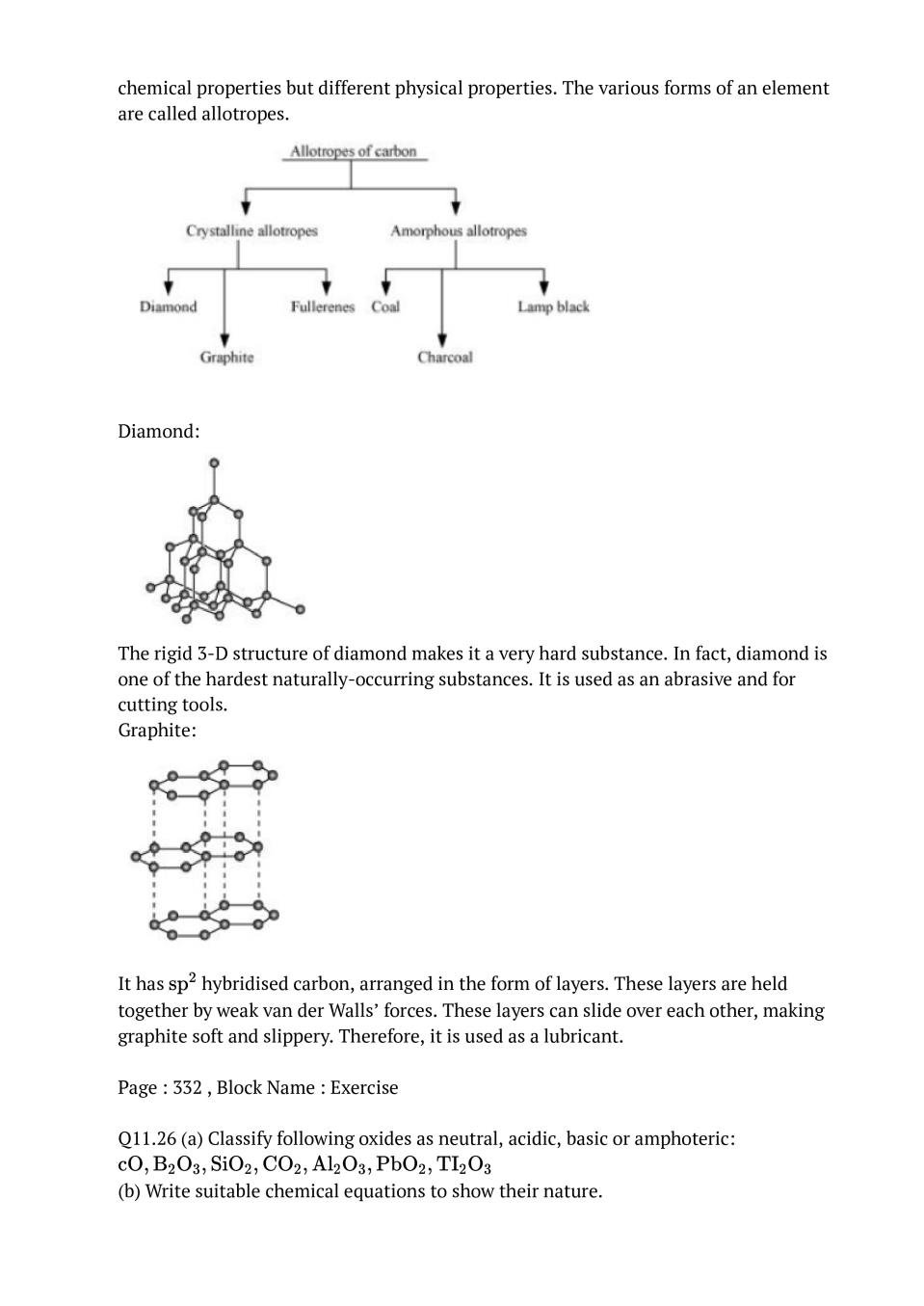
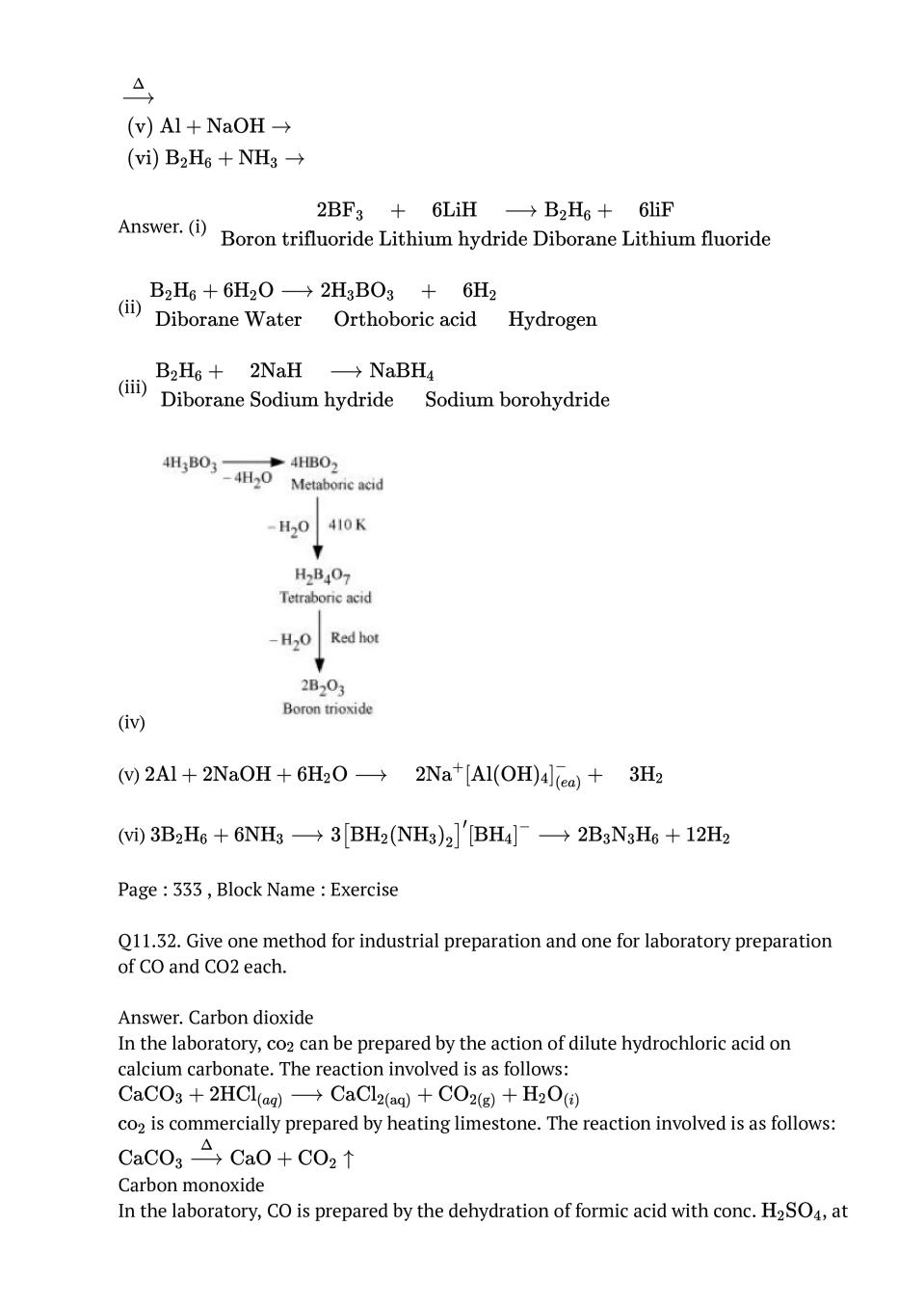
Ans : (i) B to Tl The electric configuration of group 13 elements is \( \mathrm{ns}^{2} \mathrm{np}^{1}\). Therefore, the most common oxidation state exhibited by them should be +3. However, it is only boron and aluminium which practically show the +3 oxidation state. The remaining elements, i.e., Ga, In, Tl, show both the +1 and +3 oxidation states. On moving down the group, the +1 state becomes more stable. For example, Tl (+1) is more stable than Tl (+3). This is because of the inert pair effect. The two electrons present in the s-shell are strongly attracted by the nucleus and do not participate in bonding. This inert pair effect becomes more and more prominent on moving down the group. Hence, Ga (+1) is unstable, In (+1) is fairly stable, and Tl (+1) is very stable.The stability of the +3 oxidation state decreases on moving down the group. (ii) C to Pb The electronic configuration of group 14 elements is \( n s^{2} n p^{2}\) . Therefore, the most common oxidation state exhibited by them should be +4. However, the +2 oxidation state becomes more and more common on moving down the group. C and Si mostly show the +4 state. On moving down the group, the higher oxidation state becomes less stable. This is because of the inert pair effect. Thus, although Ge, Sn, and Pb show both the +2 and + 4 states, the stability of the lower oxidation state increases and that of the higher oxidation state decreases on moving down the group.
Q.2: How can you explain higher stability of \( \mathrm{BCl}_{3}\) as compared to \( \mathrm{TICl}_{3}\) ?
Ans : Boron and thallium belong to group 13 of the periodic table. In this group, the +1 oxidation state becomes more stable on moving down the group. \( \mathrm{BCl}_{3}\) is more stable than \( \mathrm{TICl}_{3}\) because the +3 oxidation state of B is more stable than the +3 oxidation state of Tl. In Tl, the +3 state is highly oxidising and it reverts back to the more stable +1 state.
Q.3: Why does boron trifluoride behave as a Lewis acid ?
Ans : The electric configuration of boron is \( n s^{2} n p^{1}\). It has three electrons in its valence shell. Thus, it can form only three covalent bonds. This means that there are only six electrons around boron and its octet remains incomplete. When one atom of boron combines with three fluorine atoms, its octet remains incomplete. Hence, boron trifluoride remains electron-deficient and acts as a Lewis acid.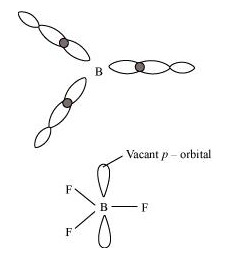
Q.4: Consider the compounds,\( \mathrm{BCl}_{3}\) and \( \mathrm{CCl}_{4}\) . How will they behave with water ? Justify.
Ans : Being a Lewis acid, BCl3 readily undergoes hydrolysis. Boric acid is formed as a result \( \mathrm{BCl}_{3}+3 \mathrm{H}_{2} \mathrm{O} \longrightarrow 3 \mathrm{HCl}+\mathrm{B}(\mathrm{OH})_{3}\) \( \mathrm{CCl}_{4}\) completely resists hydrolysis. Carbon does not have any vacant orbital. Hence, it cannot accept electrons from water to form an intermediate. When \( \mathrm{CCl}_{4}\) and water are mixed, they form separate layers. \( \mathrm{CCl}_{4}+\mathrm{H}_{2} \mathrm{O} \) -> NO reaction
Q.5: Is boric acid a protic acid ? Explain.
Ans : Boric acid is not a protic acid. It is a weak monobasic acid, behaving as a Lewis acid. \( \mathrm{B}(\mathrm{OH})_{3}+2 \mathrm{HOH} \longrightarrow\left[\mathrm{B} (\mathrm{OH})_{4}\right]^{-}+\mathrm{H}_{3} \mathrm{O}^{+}\) It behaves as an acid by accepting a pair of electrons from –OH ion.
NCERT / CBSE Book for Class 11 Chemistry
You can download the NCERT Book for Class 11 Chemistry in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Chemistry
- Click here to buy NCERT Book for Class 11 Chemistry
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Sociology
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Chemistry Chapter 11 The p-Block element. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block element appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/2Qqw04Q
https://ift.tt/3nlVtbs https://ift.tt/3nlVtbs










إرسال تعليق