NCERT Solutions Class 11 Chemistry Chapter 8 Redox Reactions– Here are all the NCERT solutions for Class 11 Chemistry Chapter 8. This solution contains questions, answers, images, explanations of the complete chapter 1 titled Redox Reactions taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Chemistry, then you must come across chapter 8 Redox Reactions After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reactions in one place.
NCERT Solutions Class 11 Chemistry Chapter 8 Redox Reactions
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Chemistry for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 8 Redox Reactions , Chemistry, Class 11.
| Class | 11 |
| Subject | Chemistry |
| Book | Chemistry Part I |
| Chapter Number | 8 |
| Chapter Name |
Redox Reactions |
NCERT Solutions Class 11 Chemistry chapter 8 Redox Reactions
Class 11, Chemistry chapter 8, Redox Reactions solutions are given below in PDF format. You can view them online or download PDF file for future use.
Redox Reactions
Did you find NCERT Solutions Class 11 Chemistry chapter 8 Redox Reactions helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Chemistry chapter 8 Redox Reactions- Video
You can also watch the video solutions of NCERT Class11 Chemistry chapter 8 Redox Reactions here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Chemistry chapter 8 Redox Reactions In PDF Format
You can also download here the NCERT Solutions Class 11 Chemistry chapter 8 Redox Reactions in PDF format.
Click Here to download NCERT Solutions for Class 11 Chemistry chapter 8 Redox Reactions
Question & Answer
Q.1: Assign oxidation number to the underlined elements in each of the following species:
\((\mathrm{a}) \mathrm{NaH}_{2} \mathrm{\underline {P}O}_{4}(\mathrm{b}) \mathrm{NaH\underline {S}O}_{4}(\mathrm{c}) \mathrm{H}_{4} \mathrm{\underline {P}}_{2} \mathrm{O}_{7}(\mathrm{d}) \mathrm{K}_{2} \mathrm{\underline {Mn}O}_{4}\)
\( (\mathrm{e})\mathrm{Ca\underline {O}}_{2}(\mathrm{f}) \mathrm{Na\underline {B}H}_{4}(\mathrm{g}) \mathrm{H}_{2} {\mathrm{\underline {S}}}_{2} \mathrm{O}_{7} \text { (h) } \mathrm{KAl}\left({\mathrm{\underline {S}O}}_{4}\right)_{2} \cdot 12 \mathrm{H}_{2} \mathrm{O}\)
Ans : (a) Let the oxidation number of P be x. We know that, Oxidation number of Na = +1 Oxidation number of H = +1 Oxidation number of O = –2 \( \mathrm{Na} \mathrm{H}_{2} \mathrm{PO}_{4}\) Then, we have \( \begin{array}{l}{1(+1)+2(+1)+1(x)+4(-2)=0} \\ {\Rightarrow 1+2+x-8=0} \\ {\Rightarrow x=+5}\end{array}\) Hence, the oxidation number of P is +5. (b) \( \stackrel{+1}{N a} H S O_{4}\) Then, we have \( \begin{array}{l}{1(+1)+1(+1)+1(x)+4(-2)=0} \\ {\Rightarrow 1+1+x-8=0} \\ {\Rightarrow x=+6}\end{array}\) Hence, the oxidation number of S is + 6. (c) \(\mathrm{H}_{4} \mathrm{P}_{2} \mathrm{O}_{7}\) Then, we have \( \begin{array}{l}{4(+1)+2(x)+7(-2)=0} \\ {\Rightarrow 4+2 x-14=0} \\ {\Rightarrow 2 x=+10} \\ {\Rightarrow x=+5}\end{array}\) Hence, the oxidation number of P is + 5. (d) \(\mathrm{K}_{2} \mathrm{MnO}_{4}\) Then, we have \( \begin{array}{l}{2(+1)+x+4(-2)=0} \\ {\Rightarrow 2+x-8=0} \\ {\Rightarrow x=+6}\end{array}\) Hence, the oxidation number of Mn is + 6. (e) \( \begin{array}{l}{+2 \quad x} \\ {\mathrm{Ca} \mathrm{O}_{2}}\end{array}\) Then, we have \( \begin{array}{l}{(+2)+2(x)=0} \\ {\Rightarrow 2+2 x=0} \\ {\Rightarrow x=-1}\end{array}\) (f) \(9 \mathrm{Na} \mathrm{B} \mathrm{H}_{4}\) Then, we have \( \begin{array}{l}{1(+1)+1(x)+4(-1)=0} \\ {\Rightarrow 1+x-4=0} \\ {\Rightarrow x=+3}\end{array}\) Hence, the oxidation number of B is + 3. (g) \( \mathrm{H}_{2} \mathrm{S}_{2} \mathrm{O}_{7}\) Then, we have \( \begin{array}{l}{2(+1)+2(x)+7(-2)=0} \\ {\Rightarrow 2+2 x-14=0} \\ {\Rightarrow 2 x=12} \\ {\Rightarrow x=+6}\end{array}\) Hence, the oxidation number of S is + 6. (h ) \( \mathrm{KAl}\left(\underline{\mathrm{SO}}_{4}\right)_{2} \cdot 12 \mathrm{H}_{2} \mathrm{O}\) Then, we have \( \begin{array}{l}{1(+1)+1(+3)+2(x)+8(-2)+24(+1)+12(-2)=0} \\ {\Rightarrow 1+3+2 x-16+24-24=0} \\ {\Rightarrow 2 x=12} \\ {\Rightarrow x=+6}\end{array}\) Or, We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have \( \begin{array}{l}{1(+1)+1(+3)+2(x)+8(-2)=0} \\ {\Rightarrow 1+3+2 x-16=0} \\ {\Rightarrow 2 x=12} \\ {\Rightarrow x=+6}\end{array}\) Hence, the oxidation number of S is + 6.
Q.2: What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results?
\( \mathrm{Kl}_{3}\)
\( \mathrm{H}_{2} \underline{\mathrm{S}_{4}} \mathrm{O}_{6}\)
(c) \( \mathrm{Fe}_{3} \mathrm{O}_{4}\)
(d) \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}\)
(e) \( \mathrm{CH}_{3} \mathrm{COOH}\)
Ans : (a) \( \mathrm{Kl}_{3}\) In \( \mathrm{Kl}_{3}\), the oxidation number (O.N.) of K is +1. Hence, the average oxidation number of I is \( -\frac{1}{3}\) . However, O.N. cannot be fractional. Therefore, we will have to consider the structure of KI3to find the oxidation states. In a \( \mathrm{Kl}_{3}\) molecule, an atom of iodine forms a coordinate covalent bond with an iodine molecule.Hence, in a \( \mathrm{Kl}_{3}\)molecule, the O.N. of the two I atoms forming the l2> molecule is 0, whereas the O.N. of the I atom forming the coordinate bond is as 1. (b) \( \mathrm{H}_{2} \underline{\mathrm{S}_{4}} \mathrm{O}_{6}\) \( \begin{array}{l}{\text { Now, } 2(+1)+4(x)+6(-2)=0} \\ {\Rightarrow 2+4 x-12=0} \\ {\Rightarrow 4 x=10} \\ {\Rightarrow x=+2 \frac{1}{2}}\end{array}\) However, O.N. cannot be fractional. Hence, S must be present in different oxidation states in the molecule.
The O.N. of two of the four S atoms is +5 and the O.N. of the other two S atoms is 0. (c) \( \mathrm{Fe}_{3} \mathrm{O}_{4}\) On taking the O.N. of O as 2, the O.N. of Fe is found to be \( +2 \frac{2}{3}\). However, O.N. cannot be fractional. Here, one of the three Fe atoms exhibits the O.N. of +2 and the other two Fe atoms exhibit the O.N. of +3. (d) \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}\) \( \begin{array}{l}{2(x)+6(+1)+1(-2)=0} \\ {\text { or, } 2 x+4=0} \\ {\text { or, } x=-2} \\ {\text { Hence, the O.N. of } C \text { is } \hat{a} \epsilon^{u} 2}\end{array}\) (e) \( \mathrm{CH}_{3} \mathrm{COOH}\) \( \begin{array}{l}{2(x)+4(+1)+2(-2)=0} \\ {\text { or, } 2 x=0} \\ {\text { or, } x=0}\end{array}\) However, 0 is average O.N. of C. The two carbon atoms present in this molecule are present in different environments. Hence, they cannot have the same oxidation number. Thus, C exhibits the oxidation states of +2 and –2 in \( \mathrm{CH}_{3} \mathrm{COOH}\).
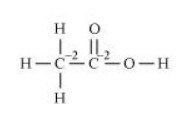
Q.3: Justify that the following reactions are redox reactions:
\( \begin{array}{l}{\text { (a) } \mathrm{CuO}(\mathrm{s})+\mathrm{H}_{2}(\mathrm{g}) \rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g})} \\ {\text { (b) } \mathrm{Fe}_{2} \mathrm{O}_{3}(\mathrm{s})+3 \mathrm{cO}(\mathrm{g}) \rightarrow 2 \mathrm{Fe}(\mathrm{s})+3 \mathrm{CO}_{2}(\mathrm{g})} \\ {\text { (c) } 4 \mathrm{BCl}_{3}(\mathrm{g})+3 \mathrm{LiAlH}_{4}(\mathrm{s}) \rightarrow 2 \mathrm{B}_{2} \mathrm{H}_{6}(\mathrm{g})+3 \mathrm{LiCl}(\mathrm{s})+3 \mathrm{AlCl}_{3}(\mathrm{s})} \\ {\text { (d) } 2 \mathrm{K}(\mathrm{s})+\mathrm{F}_{2}(\mathrm{g}) \rightarrow 2 \mathrm{K}+\mathrm{F}-(\mathrm{s})} \\ {\text { (e) } 4 \mathrm{NH}_{3}(\mathrm{g})+5 \mathrm{O}_{2}(\mathrm{g}) \rightarrow 4 \mathrm{NO}(\mathrm{g})+6 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})}\end{array}\)
Ans : (a) \(\mathrm{CuO}_{(s)}+\mathrm{H}_{2(g)} \)longrightarrow \(\mathrm{Cu}_{(s)}+\mathrm{H}_{2} \mathrm{O}_{(g)}\) Let us write the oxidation number of each element involved in the given reaction as: \( \stackrel{+2}{\mathrm{Cu}} \mathrm{O}_{(s)}+\stackrel{\theta}{\mathrm{H}_{2(\mathrm{g})}}\) -> \( \mathrm{Cu}_{(s)}+\mathrm{H}_{2} \mathrm{O}_{(g)}\) Here, the oxidation number of Cu decreases from +2 in CuO to 0 in Cu i.e., CuO is reduced to Cu. Also, the oxidation number of H increases from 0 in \( \mathrm{H}_{2}\) to +1 in \( \mathrm{H}_{2} \mathrm{O}\) i.e., \( \mathrm{H}_{2}\) is oxidized to \( \mathrm{H}_{2} \mathrm{O}\). Hence, this reaction is a redox reaction. (b) \( \mathrm{Fe}_{2} \mathrm{O}_{3(s)}+3 \mathrm{CO}_{(\mathrm{g})} \longrightarrow 2 \mathrm{Fe}_{(s)}+3 \mathrm{CO}_{2(\mathrm{g})}\) Let us write the oxidation number of each element in the given reaction as:Here, the oxidation number of Fe decreases from +3 in \( \mathrm{Fe}_{2} \mathrm{O}_{3}\) to 0 in Fe i.e., \( \mathrm{Fe}_{2} \mathrm{O}_{3}\) is reduced to Fe. On the other hand, the oxidation number of C increases from +2 in CO to +4 in \( \mathrm{CO}_{2}\) i.e., CO is oxidized to \( \mathrm{CO}_{2}\). Hence, the given reaction is a redox reaction. (c) \( 4 \mathrm{BCl}_{3}(\mathrm{g})+3 \mathrm{LiAlH}_{4}(\mathrm{s}) \rightarrow 2 \mathrm{B}_{2} \mathrm{H}_{6}(\mathrm{g})+3 \mathrm{LiCl}(\mathrm{s})+3 \mathrm{AlCl}_{3}(\mathrm{s})\) The oxidation number of each element in the given reaction can be represented as:
In this reaction, the oxidation number of B decreases from +3 in \( \mathrm{BCl}_{3}\) to –3 in \( \mathrm{B}_{2} \mathrm{H}_{6}\). i.e., \( \mathrm{BCl}_{3}\) is reduced to\( \mathrm{B}_{2} \mathrm{H}_{6}\). Also, the oxidation number of H increases from –1 in LiAlH4 to +1 in \( \mathrm{B}_{2} \mathrm{H}_{6}\) i.e., LiAlH4 is oxidized to \( \mathrm{B}_{2} \mathrm{H}_{6}\). Hence, the given reaction is a redox reaction. (d) \( 2 \mathrm{K}(\mathrm{s})+\mathrm{F}_{2}(\mathrm{g}) \rightarrow 2 \mathrm{K}+\mathrm{F}-\) The oxidation number of each element in the given reaction can be represented as:
(e) \( 4 \mathrm{NH}_{3}(\mathrm{g})+5 \mathrm{O}_{2}(\mathrm{g}) \rightarrow 4 \mathrm{NO}(\mathrm{g})+6 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})\) The oxidation number of each element in the given reaction can be represented as:
Here, the oxidation number of N increases from –3 in \( \mathrm{NH}_{3}\) to +2 in NO. On the other hand, the oxidation number of \( \mathrm{O}_{2}\) decreases from 0 in \( \mathrm{O}_{2}\) to –2 in NO and \( \mathrm{H}_{2} \mathrm{O}\) i.e., \( \mathrm{O}_{2}\) is reduced. Hence, the given reaction is a redox reaction.
Q.4: Fluorine reacts with ice and results in the change:
\( \mathrm{H}_{2} \mathrm{O}(\mathrm{s})+\mathrm{F}_{2}(\mathrm{g}) \rightarrow \mathrm{HF}(\mathrm{g})+\mathrm{HOF}(\mathrm{g})\)
Justify that this reaction is a redox reaction:
Ans : Let us write the oxidation number of each atom involved in the given reaction above its symbol as:Here, we have observed that the oxidation number of F increases from 0 in \( F_{2}\) to +1 in HOF. Also, the oxidation number decreases from 0 in \( F_{2}\) to –1 in HF. Thus, in the above reaction, F is both oxidized and reduced. Hence, the given reaction is a redox reaction.
Q.5: Calculate the oxidation number of sulphur, chromium and nitrogen in \( \mathrm{H}_{2} \mathrm{SO}_{5}, \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-} \text { and } \mathrm{NO}_{3}^{-}\) Suggest structure of these compounds. Count for the fallacy.
Ans : \( \begin{array}{l}{\text { (i) } \mathrm{H}_{2} \mathrm{SO}_{5}} \\ {2(+1)+1(x)+5(-2)=0} \\ {\Rightarrow 2+x-10=0} \\ {\Rightarrow x=+8}\end{array}\) However, the O.N. of S cannot be +8. S has six valence electrons. Therefore, the O.N. of S cannot be more than +6. The structure of \( \mathrm{H}_{2} \mathrm{SO}_{5}\) is shown as follows:\( \begin{array}{l}{\text { Now, } 2(+1)+1(x)+3(-2)+2(-1)=0} \\ {\Rightarrow 2+x-6-2=0} \\ {\Rightarrow x=+6}\end{array}\) Therefore, the O.N. of S is +6. (ii)\( \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) \( \begin{array}{l}{2(x)+7(-2)=-2} \\ {\Rightarrow 2 x-14=-2} \\ {\Rightarrow x=+6}\end{array}\) Here, there is no fallacy about the O.N. of Cr in\( \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\) The structure of \( \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}\)is shown as follows:
Here, each of the two Cr atoms exhibits the O.N. of +6. (iii) \( \mathrm{NO}_{3}^{-}\) \( \begin{array}{l}{1(x)+3(-2)=-1} \\ {\Rightarrow x-6=-1} \\ {\Rightarrow x=+5}\end{array}\) Here, there is no fallacy about the O.N. of N in \( \mathrm{NO}_{3}^{-}\) The structure of \( \mathrm{NO}_{3}^{-}\)is shown as follows:
The N atom exhibits the O.N. of +5.
NCERT / CBSE Book for Class 11 Chemistry
You can download the NCERT Book for Class 11 Chemistry in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Chemistry
- Click here to buy NCERT Book for Class 11 Chemistry
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Sociology
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Chemistry Chapter 8 Redox Reactions. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Chemistry Chapter 8 Redox Reactions appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/3xqDVQ9
https://ift.tt/3dTWEvS https://ift.tt/3dTWEvS

























Post a Comment