NCERT Solutions Class 11 Physics Chapter 13 Kinetic Theory – Here are all the NCERT solutions for Class 11 Physics Chapter 13. This solution contains questions, answers, images, explanations of the complete chapter 13 titled Of Kinetic Theory taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Physics, then you must come across chapter 13 Kinetic Theory After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Physics Chapter 13 Kinetic Theory in one place.
NCERT Solutions Class 11 Physics Chapter 13 Kinetic Theory
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Physics for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 13 Kinetic Theory , Physics, Class 11.
| Class | 11 |
| Subject | Physics |
| Book | Physics Part I |
| Chapter Number | 13 |
| Chapter Name |
Kinetic Theory |
NCERT Solutions Class 11 Physics chapter 13 Kinetic Theory
Class 11, Physics chapter 13, Kinetic Theory solutions are given below in PDF format. You can view them online or download PDF file for future use.
Kinetic Theory Download
Did you find NCERT Solutions Class 11 Physics chapter 13 Kinetic Theory helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Physics chapter 13 Kinetic Theory- Video
You can also watch the video solutions of NCERT Class11 Physics chapter 13 Kinetic Theory here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Physics chapter 13 Kinetic Theory In PDF Format
You can also download here the NCERT Solutions Class 11 Physics chapter 13 Kinetic Theory in PDF format.
Click Here to download NCERT Solutions for Class 11 Physics chapter 13 Kinetic Theory
Question & Answer
Q.1: Estimate the fraction Of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be 3Å.
Ans : \(\begin{array}{l}{\text { Diameter of an oxygen molecule, } d=3 A} \\ {=\frac{d}{2}=\frac{3}{2}=1.5 \hat{A}=1.5 \times 10^{-8} \mathrm{cm}}\end{array}\) \(\begin{array}{l}{\text { Actual volume occupied by 1 mole of oxygen gas at STP = } 22400 \mathrm{cm}^{3}} \\ {V=\frac{4}{3} \pi r^{3} \cdot N}\end{array}\) \(\begin{array}{l}{\text { Where, } N \text { is Avogadro's number }=6.023 \times 10^{23} \text { molecules/mole }} \\ {\therefore V=\frac{4}{3} \times 3.14 \times\left(1.5 \times 10^{-8}\right)^{3} \times 6.023 \times 10^{23}=8.51 \mathrm{cm}^{3}}\end{array}\) \(\begin{array}{l}{\text { Ratio of the molecular volume to the actual volume of oxygen }=\frac{8.51}{22400}} \\ {=3.8 \times 10^{-4}}\end{array}\)
Q.2: Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP : 1 atmospheric pressure, O \(^{\circ} \mathrm{C}\)). Show that it is 22.4 litres.
Ans : \(\begin{array}{l}{\text { The ideal gas equation relating pressure (P), volume }(V), \text { and absolute temperature (T) }} \\ {\text { is given as: }} \\ {P V=n R T}\end{array}\) \(\begin{array}{l}{\text { Where, }} \\ {R \text { is the universal gas constant }=8.314 \mathrm{Jmol}^{-1} \mathrm{K}^{-1}} \\ {n=\text { Number of moles }=1} \\ {T=\text { Standard temperature }=273 \mathrm{K}} \\ {P=\text { Standard pressure }=1 \mathrm{atm}=1.013 \times 10^{5} \mathrm{Nm}^{-2}}\end{array}\) \(\begin{array}{l}{\therefore V=\frac{n R T}{P}} \\ {=\frac{1 \times 8.314 \times 273}{1.013 \times 10^{5}}} \\ {=0.0224 \mathrm{m}^{3}} \\ {=22.4 \text { litres }}\end{array}\)
Q.3: \(\begin{array}{l}{\text { Figure } 13.8 \text { shows plot of } P V / T \text { versus For } 1.00 \times 10^{-3} \mathrm{kg} \text { of oxygen gas at two different }} \\ {\text { temperatures. }}\end{array}\)
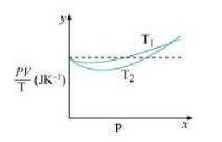 (a) does the dotted plot signify?
(b) Which is true. Tl > T2 or Tl < T2 ?
(c) What is the value Of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00 xlO-3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the v-axis? If not, what mass of
hydrogen yields the same value of p V/ T (for pressure high temperature region of the plot)?
(Molecular mass of \(\mathrm{H}_{2}=2.02 \mathrm{u}, \text { of } \mathrm{O}_{2}=32.0 \mathrm{u}, R=8.31 \mathrm{Jmol}^{-1} \mathrm{K}^{-1}\))
(a) does the dotted plot signify?
(b) Which is true. Tl > T2 or Tl < T2 ?
(c) What is the value Of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00 xlO-3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the v-axis? If not, what mass of
hydrogen yields the same value of p V/ T (for pressure high temperature region of the plot)?
(Molecular mass of \(\mathrm{H}_{2}=2.02 \mathrm{u}, \text { of } \mathrm{O}_{2}=32.0 \mathrm{u}, R=8.31 \mathrm{Jmol}^{-1} \mathrm{K}^{-1}\))
Ans : \(\begin{array}{l}{\text { (a) The dotted plot in the graph signifies the ideal behaviour of the gas, l.e., the ratio }} \\ {\frac{P V}{T} \text { is equal. } \mu R(\mu \text { is the number of moles and } R \text { is the universal gas constant) is a }} \\ {\text { constant quality. It is not dependent on the pressure of the gas. }}\end{array}\) (b) The dotted plot in the given graph represents an ideal gas. The curve of the gas at temperature Tl is closer to the dotted plot than the curve of the gas at temperature \(T_{2}\). A real gas approaches the behaviour of an ideal gas when its temperature increases. Therefore, \(T_{1} > T_{2}\) is true for the given plot. \(\begin{array}{l}{\text { (c) The value of the ratio } P V / T, \text { where the two curves meet, is } \mu R \text { . This is because the }} \\ {\text { ideal gas equation is given as: }}\end{array}\) \(\begin{array}{l}{P V=\mu R T} \\ {\frac{P V}{T}=\mu R} \\ {\text { Where, }}\end{array}\) P is the pressure T is the temperature V is the volume p is the number of moles R is the universal constant Molecular mass Of oxygen = 32.0 g \(\begin{array}{l}{\text { Mass of oxygen }=1 \times 10^{-3} \mathrm{kg}=1 \mathrm{g}} \\ {R=8.314 \mathrm{mole}^{-1} \mathrm{K}^{-1}} \\ {\quad \frac{P V}{T}=\frac{1}{32} \times 8.314}\end{array}\) \(\begin{array}{l}{=0.26 \mathrm{JK}^{-1}} \\ {\text { Therefore, the value of the ratio } \mathrm{PV} / T, \text { where the curves meet on the } y \text { -axis, is }} \\ {0.26 \mathrm{JK}^{-1}}\end{array}\) (d) If We obtain similar plots for \(1.00 \times 10^{-3}\) kg Of hydrogen, then we will not get the same value of PV/T at the point where the curves meet the V axis. This is because the molecular mass of hydrogen (2.02 u) is different from that of oxygen (32.0 u) We have: \(\begin{array}{l}{\frac{P Y}{T}=0.26 \mathrm{JK}^{-1}} \\ {R=8.314 \mathrm{Jmole}^{-1} \mathrm{K}^{-1}} \\ {\text { Molecular mass }(M) \text { of } \mathrm{H}_{2}=2.02 \mathrm{u}} \\ {\frac{P V}{T}=\mu R \text { at constant temperature }} \\ {\text { Where, } \mu=\frac{m}{M}} \\ {m=\text { Mass of } \mathrm{H}_{2}}\end{array}\) \(\begin{array}{l}{\quad m=\frac{P V}{T} \times \frac{M}{R}} \\ {=\frac{0.26 \times 2.02}{8.31}} \\ {=6.3 \times 10^{-2} \mathrm{g}=6.3 \times 10^{-5} \mathrm{kg}} \\ {\text { Hence, } 6.3 \times 10^{-5} \mathrm{kg} \text { of } \mathrm{H}_{2} \text { will yield the same value of } P V / T}\end{array}\)
Q.4: An oxygen cylinder of volume 30 litres has an initial gauge pressure of 15 atm and a temperature of 27 oc. After some oxygen is withdrawn from the cylinder, the gauge pressure drops to 11 atm and its temperature drops to 17 oc. Estimate the mass of oxygen taken out of the cylinder (R = 8.31 J \({mole}^{-1}\) \(K^{-1}\), molecular mass of 02 = 32 u).
Ans : \(\begin{array}{l}{\text { Volume of oxygen, } V_{1}=30 \text { litres }=30 \times 10^{-3} \mathrm{m}^{3}} \\ {\text { Gauge pressure, } P_{1}=15 \mathrm{atm}=15 \times 1.013 \times 10^{5} \mathrm{Pa}}\end{array}\) \(\begin{array}{l}{\text { Temperature, } T_{1}=27^{\circ} \mathrm{C}=300 \mathrm{K}} \\ {\text { Universal gas constant, } R=8.314 \mathrm{Jmole}^{-1} \mathrm{K}^{-1}} \\ {\text { Let the initial number of moles of oxygen gas in the cylinder be } n_{1}} \\ {\text { The gas equation is given as: }} \\ {P_{1} V_{1}=n_{1} R T_{1}}\end{array}\) \(\begin{array}{l}{\therefore n_{1}=\frac{P V_{1}}{R T_{1}}} \\ {=\frac{15.195 \times 10^{5} \times 30 \times 10^{-3}}{(8.314) \times 300}=18.276}\end{array}\) \(\begin{array}{l}{\text { Where, }} \\ {m_{1}=\text { Initial mass of oxygen }} \\ {M=\text { Molecular mass of oxygen }=32 \mathrm{g}} \\ {\therefore m_{1}=n_{1} M=18.276 \times 32=584.84 \mathrm{g}}\end{array}\) After some oxygen is withdrawn from the cylinder, the pressure and temperature reduces \(\begin{array}{l}{\text { Volume, } V_{2}=30 \text { litres }=30 \times 10^{-3} \mathrm{m}^{3}} \\ {\text { Gauge pressure, } P_{2}=11 \text { atm }=11 \times 1.013 \times 10^{5} \mathrm{Pa}} \\ {\text { Temperature, } T_{2}=17^{\circ} \mathrm{C}=290 \mathrm{K}}\end{array}\) \(\begin{array}{l}{P_{2} V_{2}=n_{2} R T_{2}} \\ {\therefore n_{2}=\frac{P_{2} V_{2}}{R T_{2}}} \\ {=\frac{11.143 \times 10^{5} \times 30 \times 10^{-3}}{8.314 \times 290}=13.86}\end{array}\) \(\begin{array}{l}{n_{2}=\frac{m_{2}}{M}} \\ {\text { Where, }} \\ {m_{2} \text { is the mass of oxygen remaining in the cylinder }}\end{array}\) \(\begin{array}{l}{\therefore m_{2}=r_{2} M=13.86 \times 32=453.1 \mathrm{g}} \\ {\text { The mass of oxygen taken out of the cylinder is given by the relation: }} \\ {\text { Initial mass of oxygen in the cylinder - Final mass of oxygen in the cylinder }} \\ {=m_{1}-m_{2}} \\ {=584.84 \mathrm{g}-453.1 \mathrm{g}} \\ {=131.74 \mathrm{g}} \\ {=0.131 \mathrm{kg}} \\ {\text { Therefore, } 0.131 \mathrm{kg} \text { of oxygen is taken out of the cylinder. }}\end{array}\)
Q.5: An air bubble of volume 1.0 cm³ rises from the bottom of a lake 40 m deep at a temperature of 12 °C. To what volume does it grow when it reaches the surface, which is at a temperature of 35 °C ?
Ans : \(\begin{array}{l}{\text { Volume of the air bubble, } V_{1}=1.0 \mathrm{cm}^{3}=1.0 \times 10^{-6} \mathrm{m}^{3}} \\ {\text { Bubble rises to height, } d=40 \mathrm{m}} \\ {\text { Temperature at a depth of } 40 \mathrm{m}, T_{1}=12^{\circ} \mathrm{C}=285 \mathrm{K}} \\ {\text { Temperature at the surface of the lake, } T_{2}=35^{\circ} \mathrm{C}=308 \mathrm{K}}\end{array}\) \(\begin{array}{l}{\text { The pressure on the surface of the lake: }} \\ {P_{2}=1 \text { atm }=1 \times 1.013 \times 10^{5} \mathrm{Pa}} \\ {\text { The pressure at the depth of } 40 \mathrm{m} \text { : }} \\ {P_{1}=1 \mathrm{atm}+\mathrm{d} \rho \mathrm{g}} \\ {\text { Where, }} \\ {\rho \text { is the density of water }=10^{3} \mathrm{kg} / \mathrm{m}^{3}} \\ {\therefore P_{1} \text { is the acceleration due to gravity }=9.8 \mathrm{m} / \mathrm{s}^{2}} \\ {\therefore P_{1}=1.013 \times 10^{5}+40 \times 10^{3} \times 9.8=493300 \mathrm{Pa}}\end{array}\) \(\frac{P_{1} V_{1}}{T_{1}}=\frac{P_{2} V_{2}}{T_{2}}\) Where, \(V_{2}\) is the volume Of the air bubble when it reaches the surface \(\begin{array}{l}{V_{2}=\frac{P_{1} V_{1} T_{2}}{T_{1} P_{2}}} \\ {=\frac{(493300)\left(1.0 \times 10^{-6}\right) 308}{285 \times 1.013 \times 10^{5}}} \\ {=5.263 \times 10^{-6} \mathrm{m}^{3} \text { or } 5.263 \mathrm{cm}^{3}}\end{array}\) Therefore, where, the air bubble reaches the surface, its Volume becomes 5.263 crn³.
NCERT / CBSE Book for Class 11 Physics
You can download the NCERT Book for Class 11 Physics in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Physics
- Click here to buy NCERT Book for Class 11 Physics
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Sociology
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Physics Chapter 13 Kinetic Theory. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Physics Chapter 13 Kinetic Theory appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/2QyJEmm
https://ift.tt/32NDGjY https://ift.tt/32NDGjY












إرسال تعليق