NCERT Solutions Class 11 Chemistry Chapter 13 Hydrogen– Here are all the NCERT solutions for Class 11 Chemistry Chapter 13. This solution contains questions, answers, images, explanations of the complete chapter 1 titled Hydrogen taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Chemistry, then you must come across chapter 13 Hydrogen After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrogen in one place.
NCERT Solutions Class 11 Chemistry Chapter 13 Hydrocarbons
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Chemistry for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 13 Hydrocarbons , Chemistry, Class 11.
| Class | 11 |
| Subject | Chemistry |
| Book | Chemistry Part I |
| Chapter Number | 13 |
| Chapter Name |
Hydrocarbons |
NCERT Solutions Class 11 Chemistry chapter 13 Hydrocarbons
Class 11, Chemistry chapter 13, Hydrocarbons solutions are given below in PDF format. You can view them online or download PDF file for future use.
Hydrocarbons
Did you find NCERT Solutions Class 11 Chemistry chapter 13 Hydrocarbons helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Chemistry chapter 13 Hydrocarbons- Video
You can also watch the video solutions of NCERT Class11 Chemistry chapter 13 Hydrocarbons here.
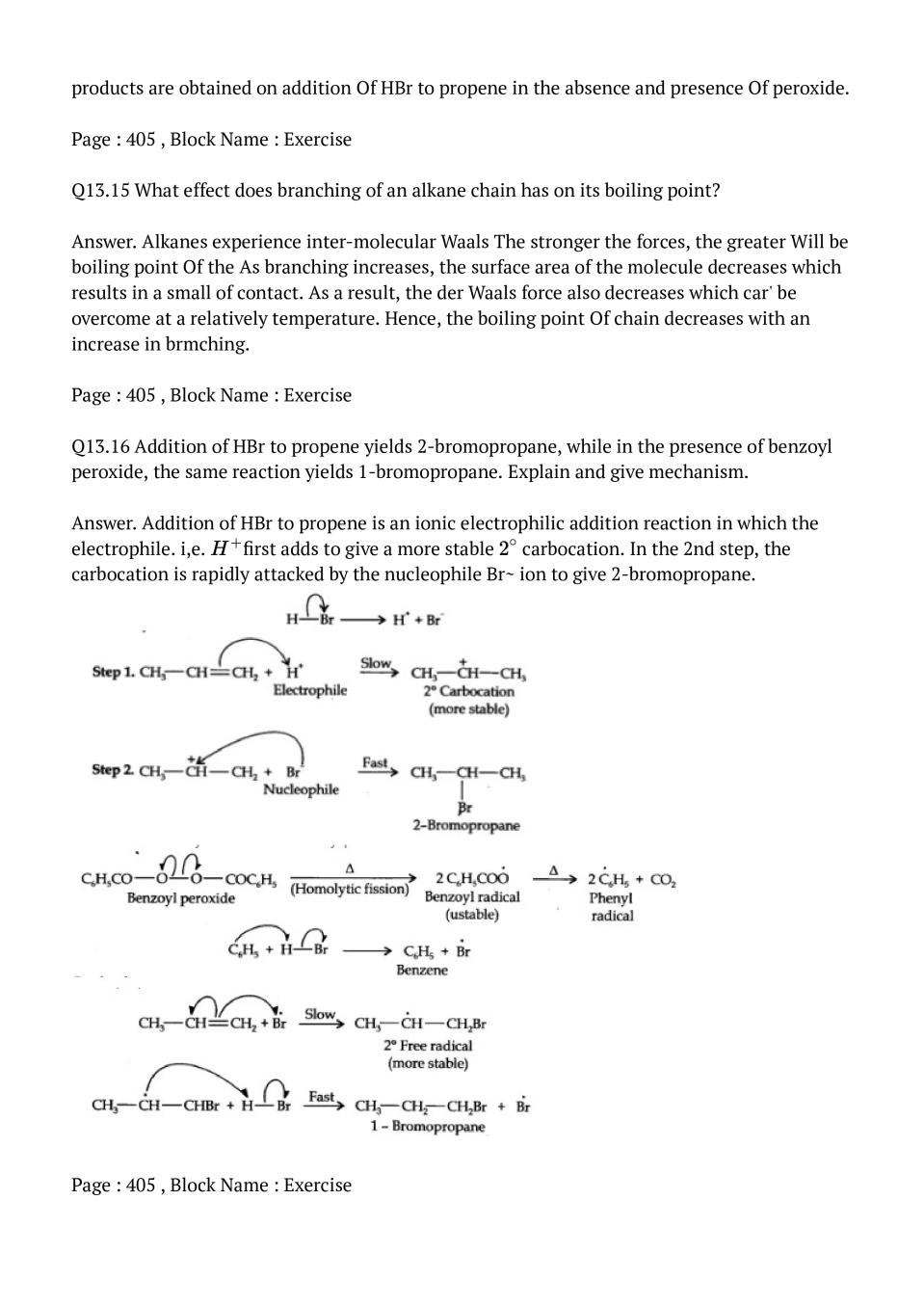
Video – will be available soon.
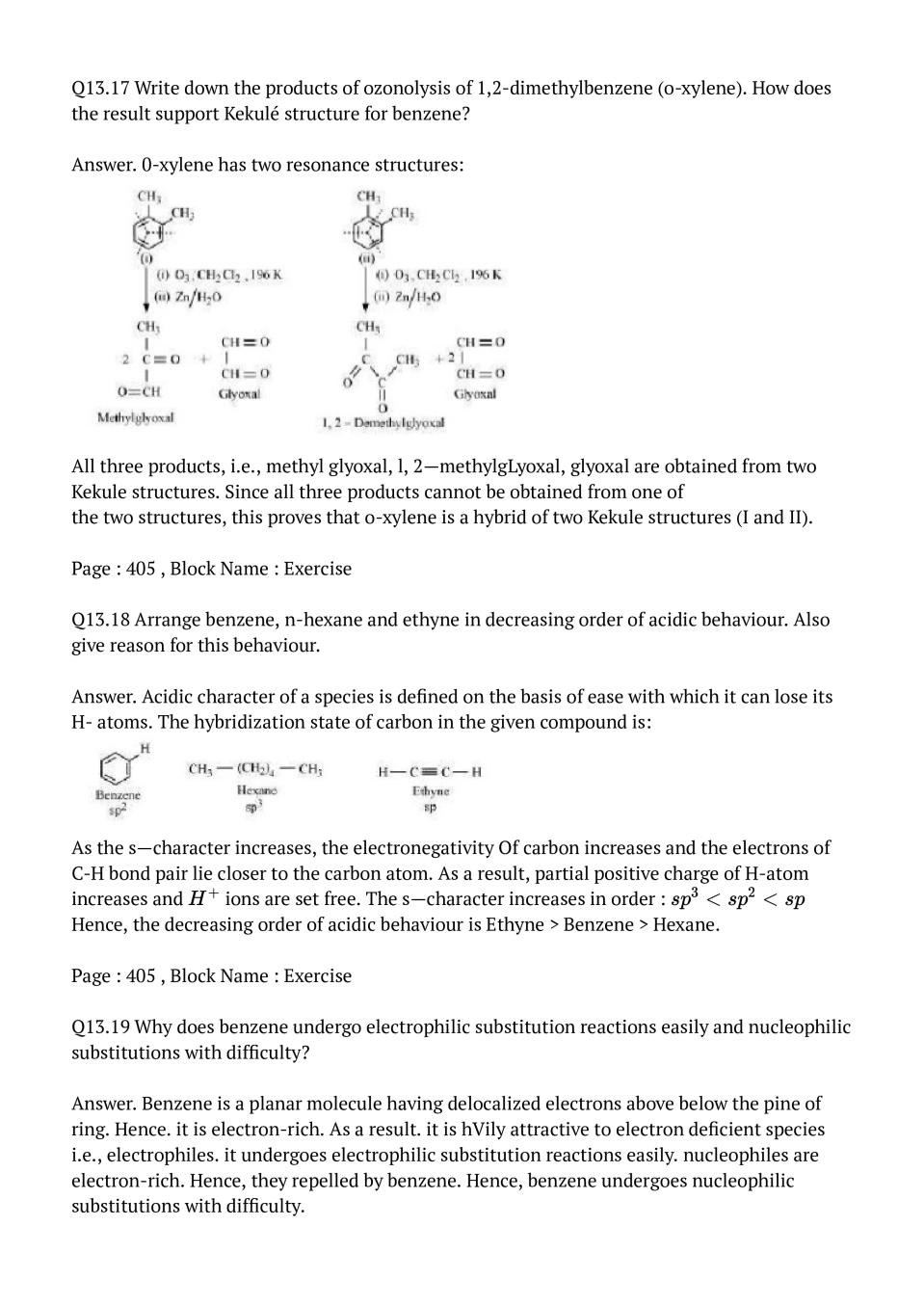
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Chemistry chapter 13 Hydrocarbons In PDF Format
You can also download here the NCERT Solutions Class 11 Chemistry chapter 13 Hydrocarbons in PDF format.
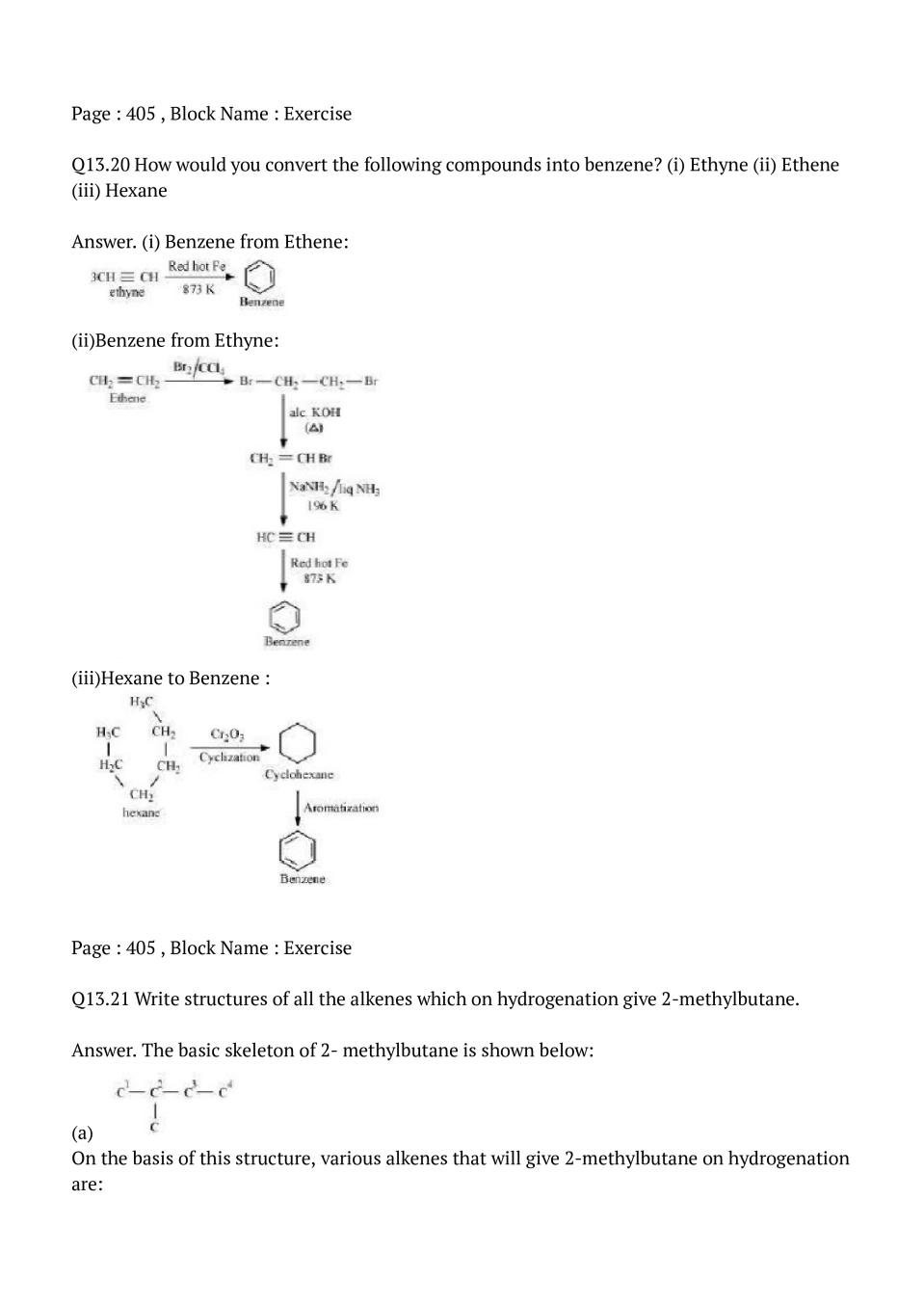
Click Here to download NCERT Solutions for Class 11 Chemistry chapter 13 Hydrocarbons
Question & Answer
Q.1: How do you account for the formation of ethane during chlorination of methane ?
Ans : The reaction begins with the homolytic cleavage of Cl — Cl bond as: Step 1: Initiation: \( \mathrm{Cl}-\mathrm{Cl} \stackrel{\mathrm{hv}}{\longrightarrow} \dot{\mathrm{Cl}}+\dot{\mathrm{C}}\) Step 2: Propagation: In the second step, chlorine free radicals attack methane molecules and break down the bond to generate methyl radicals as: \( \mathrm{CH}_{4}+\dot{\mathrm{C}} 1 \stackrel{\mathrm{h} v}{\longrightarrow} \dot{\mathrm{C}} \mathrm{H}_{3}+\mathrm{H}-\mathrm{Cl}\) These methyl radicals react with other chlorine free radicals to form methyl chloride along with the liberation of a chlorine free radical. \( \dot{\mathrm{C}} \mathrm{H}_{3}+\mathrm{Cl}-\mathrm{Cl} \longrightarrow \mathrm{CH}_{3}-\mathrm{Cl}+\dot{\mathrm{C} 1}\) Hence, methyl free radicals and chlorine free radicals set up a chain reaction. While HCI and \( \mathrm{CH}_{3} \mathrm{C}\) are the major products formed, other higher halogenated compounds are a so formed as: \( \mathrm{CH}_{3} \mathrm{Cl}+\dot{\mathrm{C}} 1 \longrightarrow \dot{\mathrm{C}} \mathrm{H}_{2} \mathrm{Cl}+\mathrm{HCl}\) \( \dot{\mathrm{C}} \mathrm{H}_{2} \mathrm{Cl}+\mathrm{Cl}-\mathrm{Cl} \longrightarrow \mathrm{CH}_{2} \mathrm{Cl}_{2}+\dot{\mathrm{Cl}}\) Step 3: Termination: Formation of ethane is a result of the termination of chain reactions taking place as a result of the consumption of reactants as: \( \dot{\mathrm{Cl}}+\dot{\mathrm{C}} \mathrm{l} \longrightarrow \mathrm{Cl}-\mathrm{Cl}\) \( \mathrm{H}_{3} \dot{\mathrm{C}}+\dot{\mathrm{C}} \mathrm{H}_{3} \longrightarrow \mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{3}\) Hence, by this process, ethane is obtained as a by-product of chlorination of methane.
Q.2: Write IUPAC names of the following compounds :
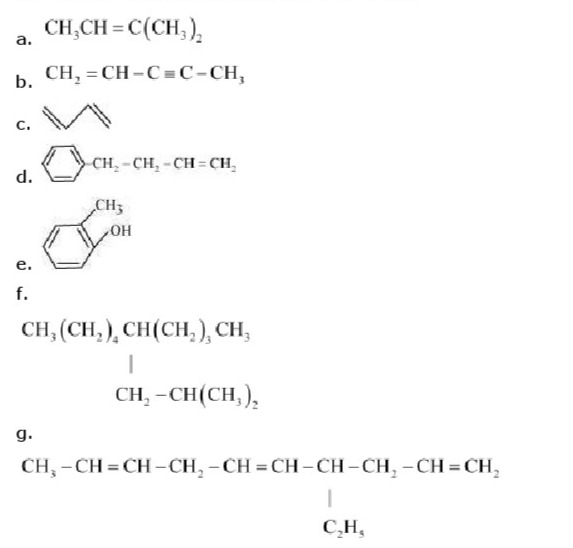
Ans : (a)IUPAC name: 2-Methylbut-2-ene (b)
IUPAC name: Pen-1 -ene-3-yne (c)
IUPAC name: 1, 3-Butadiene or Buta-1,3-diene (d)
IUPAC name: 4-Phenyl but-1-ene (e)
IUPAC name: 2-Methyl phenol (f)
IUPAC name: 5-(2-Methylpropyl)-decane (g)
IUPAC name: 4-Ethyldeca-1, 5, 8 triene
Q.3: For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated :
(a) \( C_{4}H_{8} \) (one double bond)
(b) \( C_{5}H_{8} \) (one triple bond)
Ans : (a) The following structural isomers are possible for \( \mathrm{C}_{4} \mathrm{H}_{8}\) with one double bond:The IUPAC name of Compound (I) is But-1 -ene, Compound (II) is But-2-ene, and Compound (Ill) is 2-Methylprop-1 -ene. (b) The following structural isomers are possible for \( \mathrm{C}_{5} \mathrm{C}_{8}\) with one triple bond .
The IUPAC name of Compound (I) is Pent-1 -yne, Compound (II) is Pent-2-yne, and Compound (Ill) is 3-Methylbut-1-ene.
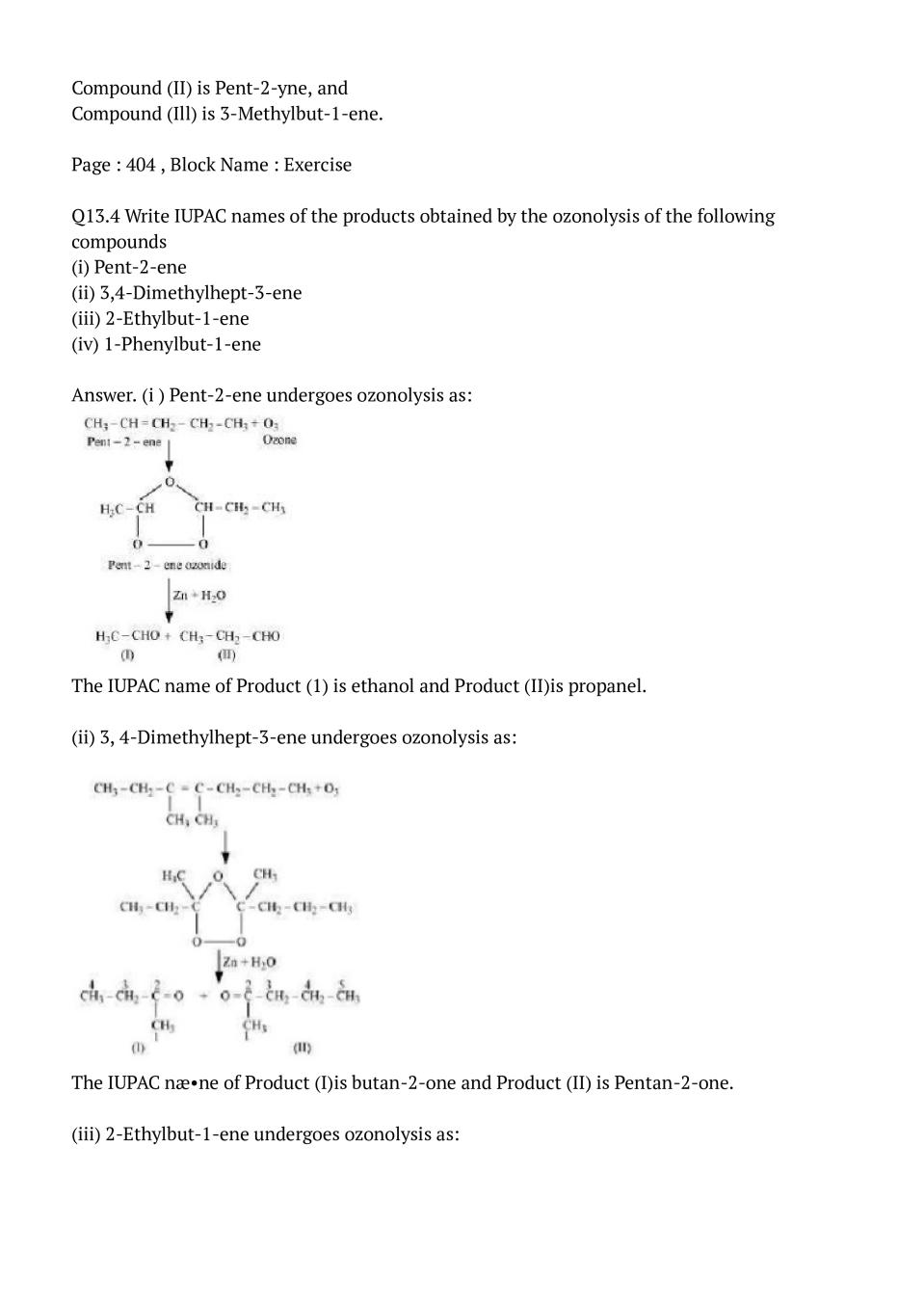
Q.4: Write IUPAC names of the products obtained by the ozonolysis of the following compounds
(i) Pent-2-ene
(ii) 3,4-Dimethylhept-3-ene
(iii) 2-Ethylbut-1-ene
(iv) 1-Phenylbut-1-ene
Ans : (i ) Pent-2-ene undergoes ozonolysis as:The IUPAC name of Product (1) is ethanol and Product (II)is propanel. (ii) 3, 4-Dimethylhept-3-ene undergoes ozonolysis as:
The IUPAC næ•ne of Product (I)is butan-2-one and Product (II) is Pentan-2-one. (iii) 2-Ethylbut-1-ene undergoes ozonolysis as:
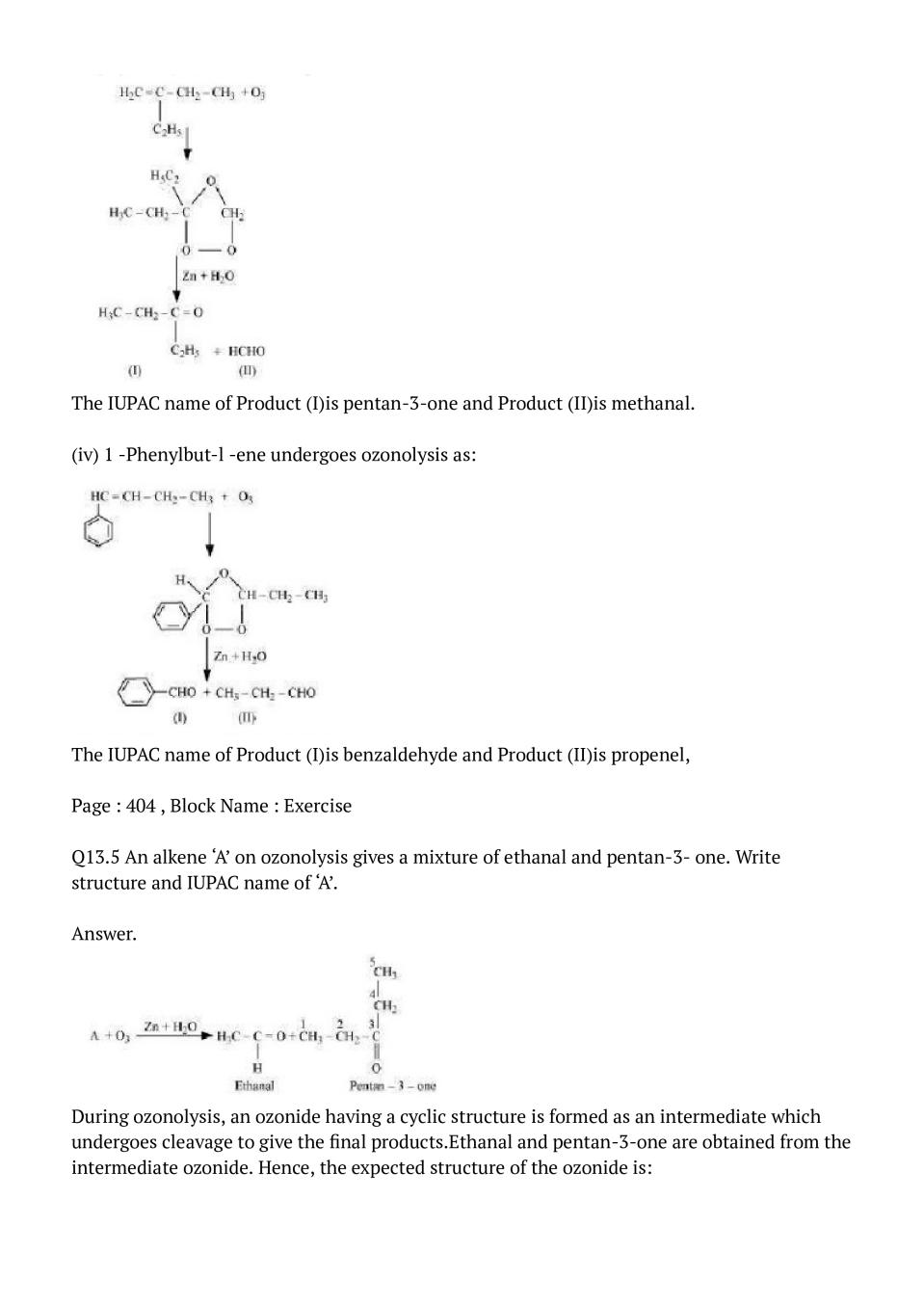
The IUPAC name of Product (I)is pentan-3-one and Product (II)is methanal. (iv) 1 -Phenylbut-l -ene undergoes ozonolysis as:
The IUPAC name of Product (I)is benzaldehyde and Product (II)is propenel,
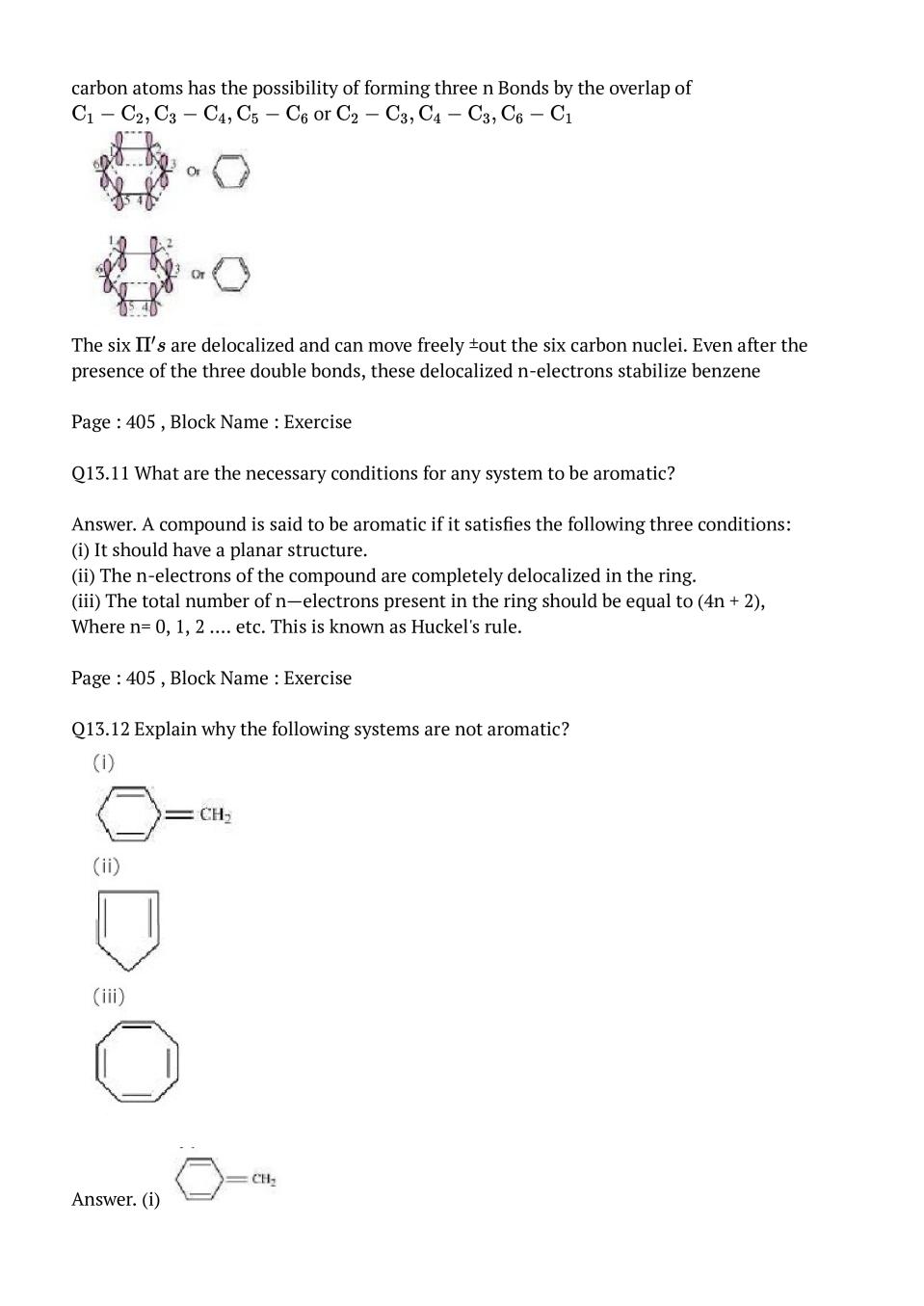
Q.5: An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
Ans :During ozonolysis, an ozonide having a cyclic structure is formed as an intermediate which undergoes cleavage to give the final products.Ethanal and pentan-3-one are obtained from the intermediate ozonide. Hence, the expected structure of the ozonide is:
This ozonide is formed as addition of ozone to The desired structure of 'A' can be obtained by the removal of ozone from the ozonide. Hence. the structural formula of 'A'
The IUPAC name of 'A' is 3-Ethylpent-2-ene.
NCERT / CBSE Book for Class 11 Chemistry
You can download the NCERT Book for Class 11 Chemistry in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Chemistry
- Click here to buy NCERT Book for Class 11 Chemistry
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Sociology
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Chemistry Chapter 13 Hydrocarbons. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Chemistry Chapter 13 Hydrocarbons appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/3vmUtXy
https://ift.tt/2R56YYY https://ift.tt/2R56YYY













Post a Comment