NCERT Solutions Class 11 Chemistry Chapter 14 Hydrogen– Here are all the NCERT solutions for Class 11 Chemistry Chapter 14. This solution contains questions, answers, images, explanations of the complete chapter 1 titled Hydrogen taught in Class 11. If you are a student of Class 11 who is using NCERT Textbook to study Chemistry, then you must come across chapter 14 Hydrogen After you have studied the lesson, you must be looking for answers of its questions. Here you can get complete NCERT Solutions for Class 11 Chemistry Chapter 14 Hydrogen in one place.
NCERT Solutions Class 11 Chemistry Chapter 14 Environmental chemistry
Here on AglaSem Schools, you can access to NCERT Book Solutions in free pdf for Chemistry for Class 11 so that you can refer them as and when required. The NCERT Solutions to the questions after every unit of NCERT textbooks aimed at helping students solving difficult questions.
For a better understanding of this chapter, you should also see summary of Chapter 14 Environmental chemistry , Chemistry, Class 11.
| Class | 11 |
| Subject | Chemistry |
| Book | Chemistry Part I |
| Chapter Number | 14 |
| Chapter Name |
Environmental chemistry |
NCERT Solutions Class 11 Chemistry chapter 14 Environmental chemistry
Class 11, Chemistry chapter 14, Environmental chemistry solutions are given below in PDF format. You can view them online or download PDF file for future use.
Environmental chemistry
Did you find NCERT Solutions Class 11 Chemistry chapter 14 Environmental chemistry helpful? If yes, please comment below. Also please like, and share it with your friends!
NCERT Solutions Class 11 Chemistry chapter 14 Environmental chemistry- Video
You can also watch the video solutions of NCERT Class11 Chemistry chapter 14 Environmental chemistry here.
Video – will be available soon.
If you liked the video, please subscribe to our YouTube channel so that you can get more such interesting and useful study resources.
Download NCERT Solutions Class 11 Chemistry chapter 14 Environmental chemistry In PDF Format
You can also download here the NCERT Solutions Class 11 Chemistry chapter 14 Environmental chemistry in PDF format.
Click Here to download NCERT Solutions for Class 11 Chemistry chapter 14 Environmental chemistry
Question & Answer
Q.1: Define environmental chemistry.
Ans : Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects, and fates of various chemical species in the environment.
Q.2: Explain tropospheric pollution in 100 words.
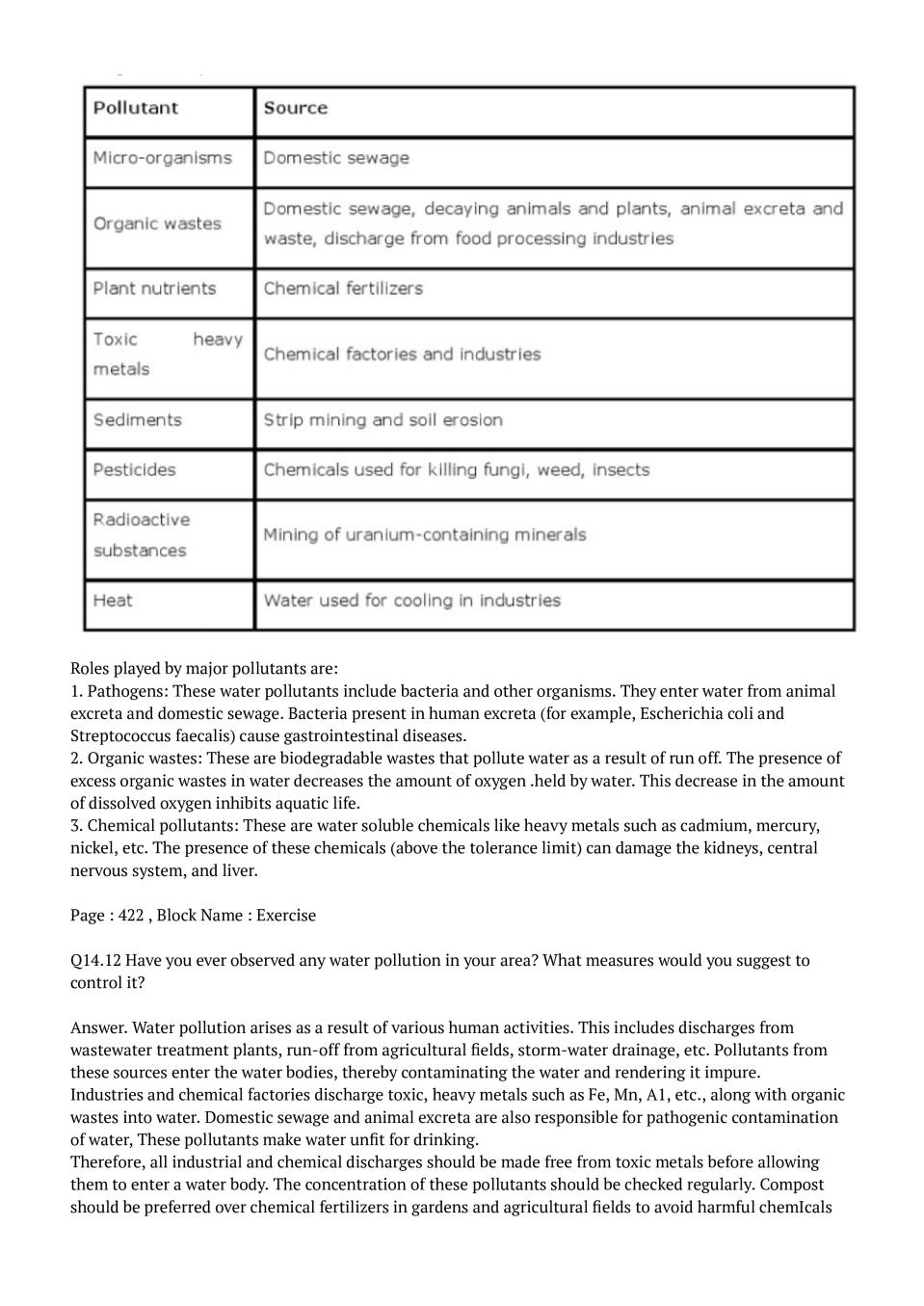
Ans : Tropospheric pollution arises due to the presence of undesirable substances in the lowest layer of the atmosphere. Oxides of sulphur, nitrogen, carbon, and hydrocarbons are the major gaseous pollutants. Oxides of sulphur (\( \mathrm{SO}_{2}\)md SO,) and nitrogen (\( \mathrm{NO}_{2}\), NO) are produced as a result of burning of fossil fuels (coal, automobile fuel). These oxides react with water in the presence of atmospheric oxygen to form nitric acid (HN03) and sulphuric acid \( \mathrm{H}_{2} \mathrm{SO}_{4}\), which leads to the formation of 'Acid rain'. \( 2 \mathrm{SO}_{2(g)}+\mathrm{O}_{2(\mathrm{g})}+2 \mathrm{H}_{2} \mathrm{O}_{(l)} \longrightarrow 2 \mathrm{H}_{2} \mathrm{SO}_{4(\mathrm{aq})}\) \(4 \mathrm{NO}_{2(\mathrm{g})}+\mathrm{O}_{2(\mathrm{g})}+2 \mathrm{H}_{2} \mathrm{O}_{(l)} \longrightarrow 4 \mathrm{HNO}_{\mathrm{y}(\mathrm{aq})}\) Acid rain causes harm to agriculture, plants, and trees, It also leads respiratory ailments. Hydrocarbons are carbon and hydrogen containing compounds that burn oxides of carbon. Hydrocarbons are carcinogenic md their products are to various to produce also major pollutants. Carbon monoxide \( \mathrm{NO}\) is poisonous in nature as it reacts with the haemoglobin of blood, which can even result in death. Though carbon dioxide \( \mathrm{CO}_{2}\) is not toxic in nature, yet it contributes towards global warming by trapping the reflected IR rays. This results in the heating up of the Earth's atmosphere, thereby leading to the melting of icebergs and glaciers. Particulates of smoke, dust, mist, and fume are harmful for human health as they are likely to block the nasal passage of a person, causing respiratory ailments. Smoke and fog combine to produce smog during a cool, humid day, thereby reducing visibility to a large extent. Photochemical smog is formed due to the presence of PAN, ozone, formaldehyde, and acrolein. It causes eye irritation, headaches, and chest pain, It also leads to the cracking of rubber and does damage to plants.
Q.3: Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Ans : Carbon dioxide \( \mathrm{CO}_{2}\) and carbon monoxide \( \mathrm{CO}\) gases are emitted during the combustion Of various fuels. Carbon monoxide is poisonous, whereas carbon-dioxide is non-toxic in nature. Carbon monoxide is poisonous because it is capable of forming a complex with haemoglobin (carboxyhemoglobin), which is more stable than the oxygen-haemoglobin complex. The concentration range of 3—4% of carboxyhemoglobin decreases the oxygen-carrying capacity of blood. This results in headaches, weak eyesight, nervousness, and cardiovascular disorders. A more increased concentration may even lead to death. Carbon dioxide is not poisonous. It proves harmful only at very high concentrations.
Q.4: List gases which are responsible for greenhouse effect.
Ans : The major greenhouse gases are: 1) Carbon dioxide \( \mathrm{CO}_{2}\) 2) Methane \( \mathrm{CH}_{4}\) 3) Water \( \mathrm{H}_{2} \mathrm{O}\) 4) Nitrous oxide \( \mathrm{NO}\) 5) Ozone \( \mathrm{O}_{3}\) 6) Chlorofluorocarbons (CFCs)
Q.5: Statues and monuments in India are affected by acid rain. How?
Ans : Acid rain is a byproduct of various human activities that leads to the emission of oxides of sulphur and nitrogen in the atmosphere. These oxides undergo oxidation and then react with water vapour to form acids. \( 2 \mathrm{SO}_{2(g)}+\mathrm{O}_{2(g)}+2 \mathrm{H}_{2} \mathrm{O}_{(l)}\) -> \( 2 \mathrm{H}_{2} \mathrm{SO}_{4(\mathrm{aq})}\) \( 4 \mathrm{NO}_{2(g)}+\mathrm{O}_{2(g)}+2 \mathrm{H}_{2} \mathrm{O}_{(l)}\) -> \( 4 \mathrm{HNO}_{3(\mathrm{aq})}\) Acid rain causes damage to buildings and structures made of stone md metal. In India, limestone is a major stone used in the construction of various monuments and statues, including the Taj Mahal. Acid rain reacts with limestone as: \( \mathrm{CaCO}_{3}+\mathrm{H}_{2} \mathrm{SO}_{4} \longrightarrow \mathrm{CaSO}_{4}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}\) This results in the loss of lustre and colour of monuments, leading to their disfiguration.
NCERT / CBSE Book for Class 11 Chemistry
You can download the NCERT Book for Class 11 Chemistry in PDF format for free. Otherwise you can also buy it easily online.
- Click here for NCERT Book for Class 11 Chemistry
- Click here to buy NCERT Book for Class 11 Chemistry
All NCERT Solutions Class 11
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Sociology
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Statistics
All NCERT Solutions
You can also check out NCERT Solutions of other classes here. Click on the class number below to go to relevant NCERT Solutions of Class 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12.
| Class 4 | Class 5 | Class 6 |
| Class 7 | Class 8 | Class 9 |
| Class 10 | Class 11 | Class 12 |
Download the NCERT Solutions app for quick access to NCERT Solutions Class 11 Chemistry Chapter 14 Environmental chemistry. It will help you stay updated with relevant study material to help you top your class!
The post NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental chemistry appeared first on AglaSem Schools.
from AglaSem Schools https://ift.tt/3aFDZSr
https://ift.tt/eA8V8J https://ift.tt/eA8V8J







Post a Comment